Abstract
The effect of pinacidil was studied on calcium-loaded rat heart mitochondria (RHM) in the presence of succinate and rotenone. In experiments with pinacidil, the swelling of these mitochondria increased in media with NH4NO3 or K-acetate, but the inner membrane potential (ΔΨmito) and the respiration in 3 or 2,4-dinitrophenol-stimulated states of these organelles decreased due to the opening of the mitochondrial permeability transition pore (MPTP) in their inner membrane. These effects were inhibited by cyclosporin A and ADP. It was concluded that the protective effect of pinacidil in the cardiac muscle under ischemia/reperfusion may be associated with both the stimulation of mitochondrial swelling and a decrease in RHM calcium overload resulted in ΔΨmito fall due to mild uncoupling effect of pinacidil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This work is a continuation of the studies started in 2012 [1]. Cardiac muscle ischemia and subsequent reperfusion leads to an increase in the Ca2+ concentration in the cytoplasm of cardiomyocytes and to calcium overload of mitochondria. This, in turn, promotes the formation of the mitochondrial permeability transition pore (MPTP) in the inner mitochondrial membrane (IMM) [2, 3]. Previously, the inner membrane MPTP opening induced by the mitochondrial calcium overload was shown in the experiments with mitochondria and cardiomyocytes in vitro, as well as in the experiments with isolated rat hearts. Experiments with isolated mitochondria and permeabilized cardiomyocytes showed that this pore opening was accompanied by mitochondrial swelling and reduction in the IMM potential (ΔΨmito). The contractile parameters of an isolated rat heart after ischemia and subsequent reperfusion were markedly improved in the experiments with modulators of mitochondrial KATP-dependent channels (mitoKATP)-pinacidil and diazoxide [4, 5]. Simultaneously, the mitochondrial calcium overload and the rate of ATP consumption in cardiomyocytes decreased. This effect of the modulators was called pharmacological preconditioning; however, the mechanism underlying this phenomenon is still not fully understood [2, 4–7]. According to one standpoint, mitoKATP modulation triggers a complex cascade preconditioning mechanism involving protein kinase C and reactive oxygen species. According to another point of view, the protective effect of these modulators is determined by their ability to mildly uncouple mitochondrial respiration and, as a result, to reduce ΔΨmito and the matrix calcium uptake, which depends on ΔΨmito.
It was found that pinacidil decreased ΔΨmito in experiments with isolated rat liver, rat brain, and rat heart mitochondria and prevented ADP-induced reduction in the IMM potential of rat heart mitochondria (RHM), energized with succinate and pyruvate [8–10]. Pinacidil reduced the accumulation of Ca2+ by mitochondria due to a decrease in Ca2+ transport into the matrix and an increase of calcium release from these organelles as well as stimulated mitochondrial swelling and the IMM proton permeability [4–6, 10]. By activating respiration in the basal and 4 states, pinacidil uncoupled oxidative phosphorylation in mitochondria, isolated from rat, mouse, and guinea pig heart [4, 8, 10–12]. Pinacidil only slightly affected state 3 respiration of these mitochondria, and ATP synthesis rate in these organelles under the influence of pinacidil changed negligibly or slightly decreased [10, 11, 13]. It was also shown that pinacidil prevented the MPTP opening in rat cardiomyocytes [14]. According to standpoint of some researchers [2, 6], there exists a certain relationship between structural elements forming mitoKATP and MPTP. To date, the effect of pinacidil on the MPTP induction in the inner membrane of mitochondria energized with succinate is poorly studied. In this regard, it was of interest to study joint effect of pinacidil and Ca2+ on the swelling, respiration, and membrane potential of succinate-energized rat heart mitochondria.
Experiments were performed on male Wistar rats weighing 200–250 g. Rat heart mitochondria (RHM) were isolated as described previously [1]. At the final stage of isolation, the organelles were resuspended in 3 mL of medium containing 300 mM sucrose and 10 mM Tris-HCl (pH 7.3). All isolation procedures were performed on ice. Protein concentration in mitochondrial samples was determined according to Bradford and was in the range of 20–30 mg/mL.
In the study we used sucrose purified from cation traces using the KU-2-8 resin filled ion exchange column. Tris-OH, KCl, carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP), oligomycin, ADP, pinacidil (Pin), succinate, rotenone, carboxyatractyloside (CAT), MgCl2, cyclosporin A (CsA), 5-hydroxydecanoate (5-HD), and MOPS were purchased from Sigma (United States). Protonophore 2,4-dinitrophenol (DNP) was of analytical grade, and the remaining reagents (NH4NO3, CaCl2, H3PO4, K-acetate, and CH3COOH) were of reagent grade.
Mitochondrial swelling was evaluated as described previously [1] using an SF-46 spectrophotometer (LOMO, Russia) at a wavelength of 540 nm at 20°C. The mitochondria were resuspended in media containing either 125 mM NH4NO3, 5 mM Tris-HCl (pH 7.3), and 1 mM Tris-PO4 (Fig. 1) or 25 mM K-acetate, 10 mM Tris-acetate (pH 7.3), and 100 mM sucrose (Fig. 2). The swelling of deenergized mitochondria in the medium containing ammonium nitrate, which dissociates in aqueous solutions to form NH3 molecule and ions \({\text{NO}}_{3}^{ - }\) and H+, makes it possible to evaluate the passive proton permeability of IMM, because this membrane is freely permeable for \({\text{NO}}_{3}^{ - }\) and NH3. All media contained 2 μM rotenone and 1 μg/mL oligomycin. Where indicated, the following additions were injected prior to mitochondria: 100 μM pinacidil, 150 μM Ca2+, 500 μM ADP, 1 μM CsA, and 300 μM 5-HD.
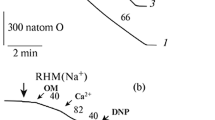
Swelling of rat heart mitochondria in the medium containing NH4NO3.The medium composition and the concentration of additions are given in the text. Additions to mitochondria: 1, no additions (control); 2, pinacidil (Pin); 3, Ca2+; 4, Pin + Ca2+; 5, Pin + Ca2+ + 5-HD; 6, Pin + Ca2+ + CsA; 7, Pin + Ca2+ + ADP; 8, Pin + Ca2+ + ADP + CsA. Additions of rat heart mitochondria (RHM) and succinate (Succ) are indicated by arrows.
Swelling of rat heart mitochondria in the medium containing K-acetate and sucrose. The medium composition is given in the text. The additions and symbols are the same as in Fig. 1.
The oxygen consumption rate (ng-atom O/min mg of protein) was evaluated polarographically using a LP-7 polarograph (Czechoslovakia) equipped with a closed Clark-type platinum electrode at 26°C in a 1.5-mL cell in medium containing 125 mM KCl, 20 mM Tris-MOPS (pH 7.3), 5 mM succinate, 1 μM rotenone, 3 mM Tris-PO4, and 0.05 mM EGTA (Fig. 3). After the addition of mitochondria (where indicated), the medium was supplemented with 150 μM ADP (ADP), 100 μM pinacidil (Pin), 2 μM CAT (CAT), 150 μM Ca2+ (Ca), 30 μM DNP (DNP), 300 μM 5-HD (5-HD), and 1 μM CsA (CsA). Given the presence of EGTA into the medium, free Ca2+ concentration was close to 100 μM. Respiration and swelling were measured at a mitochondrial protein concentration of 1 mg of per 1 mL of incubation medium.
Effect of pinacidil on the oxygen consumption rate by the rat heart mitochondria energized with succinate. Shown additions: rat heart mitochondria (RHM), ADP, pinacidil (Pin), carboxyatractyloside (CAT), Ca2+, 2,4-dinitrophenol (DNP), 5-HD, and CsA. The numerals above the curves indicate the oxygen consumption rates (ng-atom O/(min mg of protein)). The medium composition and the concentrations of additions are given in the text.
The inner membrane potential of RHM was evaluated by the change in the fluorescence intensity of safranin (conv. units) at room temperature by the standard procedure [1] (Fig. 4). Mitochondria (0.5 mg protein per 1 mL) were added to a quartz cell filled with 3 mL of a medium containing 300 mM sucrose, 10 mM Tris-HCl (pH 7.3), 3 mM Tris-PO4, 3 mM MgCl2, 2 μM rotenone, 3 μM safranine, and 1 μg/mL oligomycin. After the addition of mitochondria (where indicated), the medium was supplemented with 5 mM succinate (Succ), 100 μM pinacidil (Pin), 150 μM Ca2+ (Ca2+), and 1 μM FCCP (FCCP). The figures show the results obtained in three independent experiments.
Fluorescence of safranin in rat heart mitochondrial suspension. Arrows indicate the additions of mitochondria (RHM), succinate (Suсc), pinacidil (Pin), Ca2+, and FCCP. The medium composition and the concentrations of additions are given in the text. Experiments without additions marked as a control.
Pinacidil increased the passive proton permeability of IMM because the swelling of deenergized RHM in the medium containing NH4NO3, rotenone, and oligomycin was more pronounced than in the control experiments without pinacidil (Fig. 1, curves 1, 2). Pinacidil had no effect on the succinate-induced shrinkage of RHM preswollen in this medium in the presence of NH4NO3 as compared to the control (Fig. 1, curves 1, 2). On the other hand, the swelling of RHM energized with succinate in the experiments with Ca2+ was increased in the presence of pinacidil and did not depend on the presence of 5-HD (Fig. 1, curves 3, 4). The swelling of mitochondria, which increased in the experiments with Ca2+ and pinacidil, was followed by their shrinkage (Fig. 1, curves 6–8) in the presence MPTP inhibitors (ADP and CsA). The swelling of energized RHM in the hypoosmotic medium containing 25 mM K-acetate and 100 mM sucrose becomes possible due to K+ transport into the matrix by the electrophoretic uniport mechanism and depends on the ΔΨmito value. In this medium, the swelling of succinate-energized RHM decreased in the experiments with pinacidil as compared to the control (Fig. 2, curves 1, 2). However, calcium load of mitochondria significantly increased their swelling (curve 3), which became even more pronounced in the experiments with pinacidil (curve 4). The mitoKATP blocker 5-HD only slightly affected the combined effect of pinacidil and Ca2+ (Fig. 2a, curve 5). In the medium containing K-acetate and sucrose, this effect decreased in the experiments with CsA and ADP (Fig. 2a, curves 6, 7) and was especially noticeable in the presence of both these agents (curve 8). It is known that calcium load of mitochondria promotes the opening of MPTP in their inner membrane and is accompanied by their swelling due to the increase in the IMM permeability for ions and small molecules [2, 3]. Our data suggest that the acceleration of RHM swelling in these two media in the experiments with pinacidil and Ca2+ may be associated with the stimulation of the MPTP opening in the IMM by pinacidil.
Before the addition of ADP or uncouplers to the medium, the basal respiration of energized mitochondria depends primarily on the ion permeability of IMM. Compared to the control (Fig. 3, curve 1), the basal respiration of RHM was stimulated by pinacidil (curve 2) and did by Ca2+ to a lesser extent (curve 3). This stimulation of respiration was observed after the injection of first pinacidil and then Ca2+ (curve 4) to the medium regardless of the presence of 5-HD or CsA in it (curves 5, 6). Previously it was found [8, 15] that the pinacidil-induced activation of the basal respiration of RHM energized with pyruvate and malate was abolished after the addition of carboxyatractyloside (CAT), which fixes adenine nucleotide translocase (ANT) in the “c” conformation. Similar results were obtained in the experiments with the RHM energized with succinate (Fig. 3, curve 7). It should be noted that the addition of pinacidil and then CAT to the medium had no effect on the respiration activation by calcium ions (Fig. 3, curve 8). Thus, it can be assumed that the pinacidil-induced activation of RHM basal respiration regardless of the presence of Ca2+ in the medium is determined by the conformation of ANT. Most likely, this activation is not directly associated with the MPTP opening in the IMM.
The mitochondria respiration stimulated by uncouplers (FCCP and DNP) is determined by an activity of respiratory chain enzymes, and the respiration of phosphorylating organelles being in state 3 is additionally regulated by enzymes involved in the ATP synthesis. Unlike pinacidil, Ca2+ markedly reduced state 3 or DNP-stimulated respiration of succinate-energized RHM (Fig. 3, curves 1–3). After following addition of pinacidil and Ca2+ into the medium, state 3 mitochondrial respiration decreased regardless of the presence of the MPTP inhibitor CsA or the mitoKATP inhibitor 5-HD (Fig. 3, curves 4–6). Unlike the control (Fig. 3, curve 1), the DNP-simulated respiration of succinate-energized RHM only slightly depended on the presence of pinacidil, Ca2+, and CAT or CAT and Ca2+ (curves 2, 3, 7, 8). A slight decrease in this respiration was observed in the experiments where pinacidil and Ca2+ were present together (Fig. 3, curves 4, 5) irrespective of the presence of CsA in the medium (curve 6). The results of our experiments with the RHM, energized by succinate (Fig. 3) or by glutamate and malate [1], as well as the data of other authors [11–13] led us to conclude that pinacidil regardless of the substrate type used and in contrast to Ca2+ only slightly affected these mitochondria energetics.
Unlike [12], we did not observe any protective effect of pinacidil on the respiration of RHM loaded with calcium ions in state 3 or DNP-stimulated respiration (Fig. 3, curves 2–4). In agreement with the previous data [4, 7], we found no significant effect of pinacidil on the ΔΨmito value (Fig. 4, curve 3). After the addition of Ca2+ to the medium, a decrease in this potential was observed (Fig. 4, curve 2), which was significantly reduced in the experiments where pinacidil was added prior to calcium ions (curve 3). Our data (Figs. 3, 4) suggest that the decrease in state 3 and DNP-stimulated respiration of RHM and the ΔΨmito value in the experiments with Ca2+ were most likely due to the inner membrane MPTP opening, which was accompanied by the swelling of these organelles and cytochrome c release to the intermembrane space. On the other hand, mitoKATP modulation with pinacidil according to [2, 6] conversely prevented the opening of this pore due to the reduction in the Ca2+ entry into the matrix. These findings are consistent with the fact that the protective effect of pinacidil on the contractile parameters of an isolated rat heart is most likely due to its ability to provide a mild uncoupling effect and to reduce calcium overload of mitochondria in cardiomyocytes under the heart muscle ischemia/reperfusion like effects of another mitoKATP modulator (diazoxide).
In this study, we for the first time demonstrated the ability of pinacidil to stimulate the opening of MPTP in the low-conduction state in the inner membrane of rat heart mitochondria, energized by succinate and loaded with calcium ions. This manifested itself in an even greater swelling of mitochondria due to accelerated transport of K+ and H+ ions into the matrix as compared to similar experiments free of pinacidil. This is in good agreement with a reduction in swelling of energized RHM in the presence of MPTP inhibitors (CsA and ADP). It is assumed that the stimulation of basal mitochondrial respiration by pinacidil regardless of the substrate type used and calcium presence in the medium depends on the conformation of adenine nucleotide translocase, but it is not related to the inner membrane MPTP opening.
REFERENCES
Korotkov, S.M., Emel’yanova, L.V., Brailovskaya, I.V., and Nesterov, V.P., Dokl. Biochem. Biophys., 2012, vol. 443, pp. 113–117.
Cheng, Y., Debska-Vielhaber, G., and Siemen, D., FEBS Lett., 2005-2012, vol. 584, no. 10, pp. 2005–2012.
Halestrap, A.P. and Brenner, C., Curr. Med. Chem., 2003, vol. 10, no. 16, pp. 1507–1525.
Kowaltowski, A.J., Seetharaman, S., Paucek, P., and Garlid, K.D., Am. J. Physiol. Heart. Circ. Physiol., 2001, vol. 280, no. 2, pp. H649–H657.
Costa, A.D., Quinlan, C.L., Andrukhiv, A., et al., Am. J. Physiol. Heart. Circ. Physiol., 2006, vol. 290, no. 1, pp. H406–H415.
Ardehali, H. and O’Rourke, B., J. Mol. Cell. Cardiol., 2005, vol. 39, no. 1, pp. 7–16.
Hanley, P.J., Mickel, M., Löffler, M., et al., J. Physiol., 2002, vol. 542, pt. 3, pp. 735–741.
Kopustinskiene, D.M., Toleikis, A., and Saris, N.E., J. Bioenerg. Biomembr., 2003, vol. 35, no. 2, pp. 141–148.
Brustovetsky, T., Shalbuyeva, N., and Brustovetsky, N., J. Physiol., 2005, vol. 568, no. 1, pp. 47–59.
Holmuhamedov, E.L., Jovanović, S., Dzeja, P.P., et al., Am. J. Physiol., 1998, vol. 275, no. 5, pp. H1567–H1576.
Riess, M.L., Camara, A.K., Heinen, A., et al., J. Cardiovasc. Pharmacol., 2008, vol. 51, no. 5, pp. 483–491.
Crestanello, J.A., Doliba, N.M., Babsky, A.M., et al., J. Surg. Res., 2000, vol. 94, no. 2, pp. 116–123.
Lembert, N., Idahl, L.A., and Ammon, H.P., Biochem. Pharmacol., 2003, vol. 65, no. 11, pp. 1835–1841.
Hausenloy, D.J., Yellon, D.M., Mani-Babu, S., and Duchen, M.R., Am. J. Physiol. Heart. Circ. Physiol., 2004, vol. 287, no. 2, pp. H841–H849.
Kopustinskiene, D.M., Jovaisiene, J., Liobikas, J., and Toleikis, A., J. Bioenerg. Biomembr., 2002, vol. 34, no. 1, pp. 49–53.
ACKNOWLEDGMENTS
We are grateful to S.A. Konovalova for her assistance in measuring the safranin fluorescence in the experiments with the isolated rat heart mitochondria. Safranin fluorescence was measured using of Research Resource Center equipment for the physiological, biochemical and molecular biological studies (Sechenov Institute of Evolutionary physiology and Biochemistry, the Russian Academy of Sciences).
Funding
The study was performed in the framework of the State Assignment of the Federal Agency for Scientific Organizations (FASO) of the Russian Federation (theme no. AAAA A18-118012290142-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Korotkov, S.M., Brailovskaya, I.V., Nesterov, V.P. et al. Effects of Pinacidil and Calcium on Succinate-Energized Rat Heart Mitochondria in the Presence of Rotenone. Dokl Biochem Biophys 487, 277–281 (2019). https://doi.org/10.1134/S1607672919040070
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1607672919040070