Abstract
Polyoxymethylene/thermoplastic polyether elastomer composites were prepared by using the chain extender based on styrene, methyl methacrylate and glycidyl methacrylate copolymer as the reaction compatibilizer. The composites were fabricated by melt extrusion, and the content of thermoplastic polyether elastomer varied from 0 to 25 phr, while the amount of the chain extender was 0.2, 0.4, and finally 0.6 phr at thermoplastic polyether elastomer content 15 phr. The reactive compatibilization and the properties of the blends were studied. It was found that the viscosity of the blends increased gradually, and the notch impact strength did not change significantly after the addition of chain extender, but the tensile modulus increased significantly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Polymer modification is a cost-effective way to obtain desired polymer materials rather than synthesizing new ones [1]. There are many methods of modification, such as melt blending [2–5], reactive extrusion [6, 7], grafting modification [8, 9], filling modification [10, 11] and crosslinking [12, 13], etc. Among the above methods, melt blending is widely used because of its advantages, for example, simple equipment, easy operation and promising industrial production [14, 15]. In order to improve the properties of the blends, most scholars use many methods for polymer modification by melt blending, such as adding compatibilizer, grafting functional groups, reactive extrusion, crosslinking modification, irradiation [16–21], etc.
Polyoxymethylene (POM) resin is a kind of widely used engineering plastics, synthesized by polymerization of triformaldehyde as a monomer (trioxane) and dioxane or ethylene oxide as a comonomer [22]. Because of having strong intermolecular force and high crystallinity, POM resin has many excellent properties, such as high tensile strength, flexural modulus, and excellent self-lubricating creep. However, for the same reason, the toughness of POM is not so good, and at low temperatures (below zero centigrade) the polyacetal products will become brittle, which may limit its applications [4]. To improve the above deficiencies of POM raw resin, many toughened polyoxymethylene materials modified by different elastomers through melt blending have been reported. These elastomers could be acrylate elastomers [23, 24], poly(ethylene oxide) [25, 26], acrylonitrile-butadiene-styrene resin [27], acrylonitrile butadiene elastomer [28], methyl methacrylate-styrene-butadiene copolymer [29], polyolefin elastomer [30], thermoplastic polyurethane [31–33], etc. Among these materials, thermoplastic polyurethane is the most commonly used elastomer in the toughening modification of POM, because the hard segment of thermoplastic polyurethane is partially compatible with POM [4].
Similar to thermoplastic polyurethane, thermoplastic polyether elastomer (TPEE) materials are block copolymers which contain hard polyester segments and soft polyether segments [34, 35]. The hard segments can improve processing fluidity and rigidity of TPEE or TPEE blended materials, while soft segments contribute to increasing the toughness of these materials. Compared to thermoplastic polyurethane or other elastomers mentioned above, TPEE not only has better mechanical properties, such as tensile strength, elastic modulus, and notch impact strength, but also has better thermal stability. Therefore, it can be used to improve the impact strength and flexibility at a high or low temperature of other polymers, such as POM, PBT, PET, etc.
In this paper, in order to improve the compatibility between polyether elastomer and polyformaldehyde resin, we used chain extender KL-E4370 as the compatibilizer to prepare POM/TPEE blends and to investigate the effect of compatibilizer on the properties of blended materials systematically. Through fully studying the characterization and properties of the blends, it could be found that the melt flow performance, morphological structure, and mechanical properties of the blend materials changed after adding chain extender. For example, melt index was reduced, elastic modulus and impact strength were increased, the phase structure was more uniformed, all these indicated that epoxy chain extender was an effective compatibilizer for developing POM/TPEE blends.
EXPERIMENTAL
Materials
TPEE (CH4132) was procured from SUNPLAS Co., China. The Shall Hardness is 38; the melt flow index was 14.598 g/10 min at 190°C/2.16 kg, the brittle point was –50°C, melting point was 156°C.
POM (MC90) was manufactured by Methanol Branch of Shenhua Ningxia Coal Industry Group, China, with melt flow index of 9.142 g/10 min at 190°C/2.16 kg, the density of 1.40 g/cm3, melting point of 165–168°C, Mw = 1.84 × 105 g/mol and Ð = 2.68.
The epoxy chain extender based on styrene, methyl methacrylate and glycidyl methacrylate copolymer (KL-E4370, Scheme 1) with relative molecular weight 6500–7200 and the epoxy equivalent 280–310 g/mol produced by Shanxi Provincial Institute of Chemical Industry (Co, Ltd), China was used.

Scheme 1.
Antioxidant (Irganox1010) was purchased from Rianlon Corporation, China. The melting point was 120°C, volatile matter content was ≤0.5%, ash content was ≤0.1%.
White oil (industrial grade no. 15) was supplied by Petrochina Corporation, China.
Preparation of POM/TPEE Blends
Blends of TPEE and POM were prepared in a twin-screw extruder with the ratio of screw length to diameter equal to 40, and the diameter equal to 26 mm (ZSK26, Coperion GMBH, Germany). The blends were composed of POM MC90, TPEE CH4132, compatibilizer KL-E4370, antioxidant Irganox1010, and white oil. The specific formulation was shown in Table 1. To prevent the oxidative degradation of TPEE and POM during melting extrusion, 0.25 wt % Irganox 1010 was added to each experiment [4]. A proper amount of white oil was also needed in order to improve the dispersion of antioxidant. The operating conditions of extrusion were as follows: the temperature of each section of screw was 170/175/180/185/190/190/185/180/175; the extrusion load was 15 kg/h; and the screw extrusion speed was 300 rpm [36].
Test Methods
Melt Flow Rate (MFR) of the POM/TPEE blends was measured by Melt Index Instrument (JJ‑Test Materials Testing Industry Co. LTD, China, Type: MFI-2322). According to the ISO standard test method for Melt Index (ISO 1133), the test temperature was 190°C and the test sample load was 2.16 kg [37].
The tensile and bending test samples were molded by injection using HAAKE MiniJet-II. The mold model of the tensile sample was 557-2298; the mold model of the bending sample was 557-2296: 80 × 10 × 4 mm. The tensile properties of the specimens were tested according to ISO527-2 and the bending properties according to ISO178:2001 using Universal Material Testing Machine (INSTRON 5966) [38]. The stretching rate was 50 mm/min, and the bending speed was 2 mm/min. An Impact testing machine (INSTRON CEAST 9050) was used for measuring notched charpy strength (the mold model of the Impact testing sample is 557-2296: 80 × 10 × 4 mm). All of the test samples were placed in the Constant Climate Chambers (BINDER, Model KBF 240) for 40 h state adjustment at 23 ± 2°C and 50 ± 5% relative humidity. The above mechanical tests were also performed at 23 ± 2°C and 50 ± 5% relative humidity.
For the DSC analysis, a NETZSCH DSC 200F3 with nitrogen as the purge gas was used. The samples were heated from 30 to 200°C and then maintained at 200°C for 5 min to eliminate their thermal history. The samples were subsequently cooled down to 30°C and stayed in this temperature for 5 min before being reheated back to 200°C [39].
FTIR spectra of the specimens were carried out on a BRUKER Vertex 70 FTIR spectrometer (Bruker Daltonics Inc.). The test specimens were made into small pieces of films by using the melt-pressing method operated on the thermal platform [40, 41].
The sample was fractured in liquid nitrogen, etched by the mixed solvent (the ratio of acetone to chloroform was two to three) at 40°C for 8 h, and then dried in a vacuum at 60°C [4]. The surface of the sample was coated with a thin layer of gold. Phenom PRO PW-100-016 scanning electron microscope was used at 10 kV [42].
Dynamic mechanical properties of the samples (60 × 10 × 3 mm) were measured under three-point bending mode by a NETZSCH 242E DMA system in a nitrogen atmosphere (50 mL/min). The measured frequency was 5 Hz, and the temperature scan was carried out from –100 to 100°C at a heating rate of 3 deg/min [43].
A polarized light microscope (PLM) (ZEISS Scope.A1, Germany), equipped with a Linkam LTS 420 hot stage (Linkam Scientific Instruments Ltd., Tadworth, UK) was used to study the crystallization process of the POM/TPEE blends. The samples were heated at 200°C for 2 min, then quickly cooled (10 deg/min) to the isothermal crystallization temperature of approximately 147°C. The growth of the spherulites was observed during crystallization using a camera at 200× magnification [44].
The rheological analysis was performed using an Anton Paar MCR 302 rotational rheometer. The steel plate diameter was 25 mm, and the testing gap was 1 mm. For performing the frequency sweep tests, the angular frequency was varied from 0.01 to 100 Hz, and the testing temperature was 180°C [45, 46].
RESULTS AND DISCUSSION
Usually, if two different thermoplastic materials are mixed into a modified compound by melt blending, the MFR of the above product will be between the two raw materials and is related to the ratios of them. As shown in Table 2, with the increase of CH4132 content, the MFR of samples from 1 to 5 is increased gradually, and is between the POM’s and TPEE’s. That is because under the same conditions, the melt flow performance of CH4132 is better than that of MC90, so the contribution of CH4132 to the MFR of the composite is higher than MC90. Comparing the MFR of samples 3, 6, 7, and 8, the result can be found that after using KL-E4370 as the compatibilizer, the MFR of POM/TPEE blends will be decreased obviously and the more KL-E4370 is added, the smaller the MFR of samples becomes. This result can be interpreted as the crosslinking reaction of the epoxy groups of KL-E4370 with hydroxyl end groups of POM and TPEE producing new larger molecular structures that increase the compatibility between POM and TPEE.
Mechanical properties, especially the tensile strength and notched impact strength are the key factors that influence the application of POM materials. The above properties of neat POM and POM/TPEE blends are shown in Fig. 1. The tensile strength of the blends decreases with the TPEE content increased. On the other hand, the notched impact strength of the blends increases with the increase of TPEE content, indicating the excellent toughing effect of TPEE on POM. It is obvious that, because the soft polyether segment of TPEE is amorphous, its softness leads to a reduction in the stiffness and strength of the POM matrix. In addition at the same condition of 15 phr TPEE content, sample 7 has the best mechanical properties, which is better than samples 3, 6, or 8. This indicates that there exists a loading limit of KL‑E4370, and this is why the mechanical properties of sample 8 with 0.6 KL-E4370 is not as good as of sample 7.
(1) Tensile strength and (2) impact strength of the POM/TPEE blends. Numeration of the samples is defined in Table 1.
The influence of the content of CH4132 and KL‑E4370 on the crystallization behavior of the POM blends are evaluated through DSC analysis. First, for the TPEE/POM blends without adding KL-E4370, the crystallization temperature shifts toward the low-temperature zone with the increase of CH4132 content from 143 to 138°C (Fig. 2). This is because the molecular chain structure of MC90 is more flexible, and the molecular chain segments are more easily oriented and crystallized. However, for TPEE molecules with hard segments and soft segments, the hard segment is crystalline, but the soft segment is amorphous that will affect the crystallization temperature of blends. Second, after adding KL-E4370 as a compatibilizer, the crystallization temperature of blends decreases, and the decreased value enhances with the amount of KL‑E4370 from 0.2 to 0.6 phr. This indicates that the epoxy-functional group of KL-E4370 reacts with the functional groups of POM and TPEE, and KL-E 4370 plays a role of reactive compatibilization during TPEE/POM blends preparation.
Figure 3 shows the FTIR spectra of POM, TPEE, KL-E4370, and TPEE/POM materials. From the FTIR spectra of TPEE/POM blends, we can find the band corresponding to stretching vibration of the carboxyl group, which is attributed to the overlapped bands at 1720 cm–1 for TPEE [4] and 1726 cm–1 for KL-E4370. In addition, the characteristic epoxy functional groups [47] of KL-E4370 assigned to bands at 908 and 846 cm–1 are not found in these FTIR spectra, indicating that the reaction of epoxy groups in KL-E4370 with the end groups of POM and TPEE was successful.
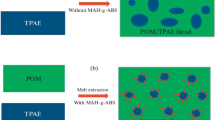
The morphological structures of fractured surface TPEE/POM blends etched by solvent are shown in Fig. 4. The images exhibit the cross-sectional morphology of the TPEE/POM blend samples with the same content of TPEE but different KL-E4370 content accordingly. The cross-section voids are formed after the TPEE elastomeric particles have been etched. In the absence of compatibilizer (Fig. 4a), it can be found that TPEE particles with different particle sizes are irregularly dispersed in the POM matrix. That is to say, only adopting the method of physical melt blending, TPEE can not be uniformly dispersed in the POM matrix. However, after adding compatibilizer such as KL-E4370, the phase state of the blend has been improved obviously (Figs. 4b–4d). It can be seen that the TPEE particles are dispersed uniform in the POM phase. It is worth mentioning that Fig. 4c clearly shows that after adding of 0.4 phr compatibilizer, the TPEE and POM phases in the blends are intertwined with each other, then forming a interpenetrating polymer network structure.
The dynamic mechanical analysis curves of TPEE, POM, and TPEE/POM blends are shown in Fig. 5. It can be seen that the E ' value of the pure POM is the highest in the test range of –100 to +100°C, but the pure TPEE has the smallest value of E '. For the TPEE/POM blends without using KL-E4370 as a compatibilizer, the E ' value is between the POM and TPEE, and it decreases with the increase of TPEE content in the above blends. The E ' value of blends containing 15 phr TPEE increases with growth of KL‑E4370 content from 0.2 to 0.6 phr.
From Fig. 5b it can be seen that the glass transition temperature Tg and tan δ of the pure TPEE are higher than that of pure POM, and the tan δ of the TPEE/POM blends increases regularly with the increase of TPEE content. The Tg value of the blends drifts slightly to the right side of the transverse axis with the increase of TPEE content. This is due to the better flexibility of POM than that of TPEE, so the Tg of POM is lower than that of TPEE. In addition, TPEE molecule contains amorphous polyether soft segments which show the viscoelasticity of rubber materials, so the tanδ of TPEE is much larger than for POM and the blends. Addition of KL-E4370 slightly affects the tanδ value of blends containing 15 phr TPEE.
As we now know that the brittleness of POM is mainly caused by its high crystallinity and large spherulite size [48]. In order to obtain POM materials with good toughness, the most effective way to decrease spherulite size and crystallinity of POM resin is using functional elastomers to modify POM. In this paper, TPEE is used as a toughening elastomer and KL-E4370 as a reactive compatibilizer to prepare TPEE/POM blends. Figure 6 presents the polarizing optical micrographs of pure POM, TPEE and TPEE/POM blends. The typical crystalline morphology of POM is shown in Fig. 6a, which has large and complete spherulites, but for TPEE no crystallization is found because the molecule of TPEE contains a large number of amorphous polyether segments which affect the crystallization properties of TPEE (Fig. 6b). After addition of 15 phr TPEE, the spherulite size and crystallinity of TPEE/POM blend decrease significantly (Fig. 6c). It was found that using KL-E4370 as compatibilizer can significantly increase the modulus and impact strength of the blends. However, how does this compatibilizer affect the crystallization of blends? This can be explained by Figs. 6d–6f. After adding of 0.2, 0.4, and 0.6 phr of compatibilizer, more amorphous regions appeared in the blends and the crystallinity is smaller than for TPEE/POM blend without KL-E4370. All these can be explained by reactive compatibilization of KL-E4370 with TPEE and POM. The result is that the compatibility between TPEE and POM is improved, and the crystalline orientation of the POM molecular segment is reduced.
Rotational rheological analysis [36] can truly reflect the processing performance of materials under melting-shearing conditions. As we now know, the shear action of material in rotational rheological test is a dynamic process and the dynamic modulus G of a material is a complex modulus. The modulus is composed of energy storage modulus G ' and loss modulus G ' ': G = G ' + iG ' '. The storage modulus versus loss modulus of the blend samples obtained under melt-shearing conditions are shown in Fig. 7. It can be concluded from the graph that in the high frequency region, the four curves are close to each other and have basically the same slope due to the shear thinning of polymer, which means that the samples have similar viscoelasticity. However, in the low frequency region, the results are completely different: the curves gradually separate and have different slopes. The slope order of the corresponding curves is as follows: sample 7 > sample 8 > sample 6 > sample 3 (the samples are defined in Table 1). These results also show that the compatibility of POM/TPEE blends can be improved by adding KL-E4370 to some extene due to upper load limit of KL-E4370.
CONCLUSIONS
The study shows us that using TPEE as a toughened elastomer to modify POM resin is an excellent way to improve the toughness of POM. With the increase of TPEE content, the toughness of modified POM increased gradually. Besides above, in order to improve the compatibility between TPEE and POM, the KL-E4370 was used as a compatibilizer. Through MFR, mechanical testing, DSC, DMA, FTIR, SEM, PLM, and rotational rheology analysis and characterization of TPEE/POM blends with addition of KL‑E4370, it was found that 1) the MFR of the blends decreased gradually; 2) the tensile strength and notched impact strength of the blends was significantly improved; 3) the crystallization temperature of blends decreased, and the decreased value enhanced with the rise of amount of KL-E4370; 4) TPEE phase was more evenly dispersed in POM phase; 5) more amorphous regions appeared in the blends; 6) the tensile modulus and loss modulus of blends were all increased with the amount of compatibilizer KL‑E4370 accordingly. All the above improvements confirmed that KL-E4370 was an effective reactivity compatibilizer for modified POM materials with TPEE.
REFERENCES
M. Droscher, G. Lieser, H. Reimann, and G. Wegner, Polymer 16, 497 (1975).
Y. Li and H. Shimizu, Macromol. Biosci. 7, 921 (2007).
T. McNally, W. R. Murphy, C. Y. Lew, R. J. Turner, and G. P. Brennan, Polymer 44, 2761 (2003).
W. Fang, X. Fan, H. Jiao, Z. Jin, W. Yuan, A. Zhang, and T. Zhou, Polym. Sci., Ser. A 61, 890 (2019).
Z. Yu, J. Wang, P. Li, D. Ding, X. Zheng, C. Hu, Z. Gao, T. Hu, X. Gong, and C. Wu, Polymers 12, 584 (2020).
E. M. Alosime, G. A. Edwards, and D. J. Martin. J. Appl. Polym. Sci. 135, 46369 (2018).
S. Siyamak, B. Laycock, and P. Luckman, Carbohydr. Polym. 227, 1 (2018).
Y. Wang, Q. Ni, and Z. Liu, J. Polym. Res. 18, 2185 (2011).
E. Rodríguez-Alba, L. Huerta, A. Ortega, and G. Burillo, ChemistrySelect 4, 7759 (2019).
Y. Yang, L. Zhang, Z. Xiong, Z. Tang, R. Zhang, and J. Zhu, Sci. China: Chem. 59, 1355 (2016).
M. Pluta, Polymer 45, 8239 (2004).
C. Zhang, J. Chang, H. Zhang, C. Li, and H. Zhao, Polymers 11, 1624 (2019).
S. S. Liu, H. Y. Ge, Y. Zou, and J. Chen, Mater. Sci. Forum 944, 521(2019).
E. L. Moya, R. Van Grieken, A. Carrero, and B. Paredes, Macromol. Symp. 321, 46 (2012).
C. Liang, C. Hu, Y. Zheng, K. Yan, and X. Zhu, Polym. Compos. 39, 762 (2018).
C. W. Macosko, P. Guégan, and A. K. Khandpur, Macromolecules 20, 5590 (1996).
H. K. Jeon, J. Zhang, and C. W. Macosko, Polymer 46, 12422 (2005).
S. C. G. Da Costa, M. D. C. Goncalves, and M. I. Felisberti, J. Appl. Polym. Sci. 72, 1827 (1999).
A. L. Hou and J. P. Qu, Polymers 11, 771 (2019).
B. -U. Nam and Y. Son, J. Appl. Polym. Sci. 137, 49011 (2020).
A. B. Balaji, C. T. Ratnam, and M. Khalid, Radiat. Phys. Chem. 141, 179 (2017).
M. Droscher, G. Lieser, H. Reimann, and G. Wegner, Macromol. Chem. Phys. 177, 1695 (1976).
X.-C. Ren, L. Chen, H.-J. Zhao, Y. Dan, and X.-F. Cai, J. Macromol. Sci. 46, 411 (2007).
G. Wu, F. Yang, S. Zhang, and X. Ren, J. Appl. Polym. Sci. 123, 2609 (2012).
Y. Liu, T. Zhou, Z. Chen, L. Li, Y. Zhan, and F. Liu, Polym. Adv. Technol. 25, 760 (2014).
J. B. Enns and R. Simha, J. Macromol. Sci. 13, 25 (1976).
A. K. Das, S. Suin, N. K. Shrivastava, S. Maiti, J. K. Mishra, and B. B. Khatua, Polym.Compos. 35, 274 (2014).
G. Q. Pan, J. Y. Chen and H. L. Li, Plast. Rubber Compos. 36, 291 (2007).
X. Wang and X. Cui, Eur. Polym. J. 41, 871 (2005).
W. Yang, X. Lun Wang, X. Yan, and Z. Guo, Polym. Eng. Sci. 57, 1119 (2017).
X. Gao, C. Qu, and Q. Fu, Polym. Int. 53, 1666 (2004).
M. Mehrabzadeh and D. Rezaie, J. Appl. Polym. Sci. 84, 2573 (2001).
M. Hosseinabadi, M. Ghetmiri, A. S. Dindarloo, and Y. Jahani, Polym. Sci., Ser. A 60, 816 (2018).
S. H. Cho, Y. J. Jang, D. M. Kim, T. Lee, D. Lee, and Y. Lee, Polym. Eng. Sci. 49, 1456 (2009).
J.-M. Lee, B.-H. Choi, J.-S. Moon, and E.-S. Lee, Polym. Test. 28, 854 (2009).
Y. Kismet, Pamukkale Univ. J. Eng. Sci. 22, 241 (2016).
J. Wang and Q. Dou, Polym. Int. 57, 233 (2008).
V. Everaert, G. Groeninckx, M. H. J. Koch, and H. Reynaers, Polymer 44, 3491 (2003).
N. V. Ramirez, M. Sanchez-Soto, and S. Illescas, Polym. Compos. 32, 1584 (2011).
Y. Duan, H. Li, L. Ye, and X. Liu, J. Appl. Polym. Sci. 99, 3085 (2006).
A. K. Das, S. Suin, N. K. Shrivastava, S. Maiti, J. K. Mishra, and B. B. Khatua, Polym. Compos. 35, 273 (2014).
S. Bai and Q. Wang, J. Vinyl Addit. Technol. 22, 479 (2016).
D. Czarnecka-Komorowska and T. Sterzynski, Polymers 10, 203 (2018).
W. Yang, X.-L. Wang, J. Li, X. Yan, S. Ge, S. Tadakamalla, and Z. Guo, Polym. Eng. Sci. 58, 1127 (2017).
J. Andrzejewski and K. Skórczewska, Polymers 12, 307 (2020).
L. B. Canto and L. A. Pessan, Polym. Test. 21, 35 (2002).
X. Gao, C. Qu, and Q. Fu, Polym. Int. 53, 1666 (2004).
I. Alig, P. Pötschke, D. Lellinger, T. Skipa, S. Pegel, G. R. Kasaliwal, and T. Villmow, Polymer 53, 4 (2012).
Funding
This work was supported by the 2018 Key R&D Project of Ningxia Hui Autonomous Region (2018BDE02040), China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wei Fang, Fan, X., Jin, Z. et al. Reactive Compatibilization of Multi-Hydroxy Functional Compound Based on Polyoxymethylene/Thermoplastic Polyether Elastomer Blends. Polym. Sci. Ser. B 62, 724–733 (2020). https://doi.org/10.1134/S1560090420060032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090420060032