Abstract
Here we report the synthesis and viscoelastic properties of smart hydrogels based on N-isopropylacrylamide, cationic (3-acrylamidopropyl)trimethylammonium chloride and nanoclay. Hydrogels were prepared by aqueous free radical polymerization. The effect of some salts, N,N '-methylenebisacrylamide, (3-acrylamidopropyl)trimethylammonium chloride and nanoclay content on the viscoelastic properties was studied. Also volume phase transition temperature and thermal behavior of the prepared hydrogels were investigated. Synthetic nanoclay and anionic groups of the bind to the cationic co-monomer units and significantly affected the viscoelasticity and thermal properties of the hydrogels. DSC measurements showed that the volume phase transition temperature of hydrogels depended on nanoclay content. By introducing of co-monomer, 250 mM solution of NaCl 15 mM bis(trifluoromethane)sulfonimide lithium salt (LiNTF2) and laponite nanoclay, a 10, 4, and 3.8 fold increase in elastic module was obtained, respectively, compared to that of the homopolymer PNIPAM hydrogel. With a temperature change from 20 to 45°C for the homopolymer PNIPAM hydrogel in 15 mM LiNTF2, the elastic module grew 5 times larger.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Hydrogels are network polymers that with absorbing water or biofluids are swelled, which then gain high flexibility [1]. Hydrogels have been used for agricultural and horticultural purposes for many years, but due to their other properties gradually they have found other uses [2, 3]. These materials exist in nature (gelatin and agar) or are prepared synthetically [4]. Depending on the structure of the hydrogels, these materials are sensitive to physical (temperature, pressure, light, and electric field), chemical (pH, glucose and oxidants) and biochemical (antigen, enzyme) factors [5]. These properties of hydrogels have made them widely used in medical, pharmacology, agricultural, sensors, elimination of environmental pollutants and for many other applications [5].
Synthesis of hydrogels is usually done using hydrophilic monomers having carbon-carbon double bonds by radical polymerization. In the manufacture of hydrogels, cross-links are also formed with an appropriate amount of cross-linking agent. Another method of preparing hydrogels is to modify polymers by grafting or functionalizing them with hydrophilic portions [6, 7]. Hydrogels are cross-linked physically and chemically. Physical cross-linkage may occur by hydrogen bonds, from amphiphilic graft and block polymers, by crystallization and ionic or protein interaction. Chemical cross-linking occurs by complementary groups chemical reaction, high energy radiation, free radical polymerization or using enzymes [4].
The physical and mechanical properties of hydrogels as well as the rate of response to stimuli such as temperature, pH, pressure, electrical current, etc. are of particular importance in the manufacture of hydrogels [8–11]. One of the compounds used in the manufacture of hydrogels is N-isopropylacrylamide and its derivatives, which provide good mechanical properties in the hydrogel and can provide response to the change of the temperature [12–14]. Also the use of nanoparticles to enhance the performance of hydrogels has been interested to researchers and hydrogels with new capabilities have been synthesized [15–17].
The use of hydrogels in medicine and tissue engineering is increasing due to their biocompatibility. Hydrogel scaffolds are one of the applications of hydrogels whose biological and mechanical properties are very important in their performance. Viscoelastic behavior is one of the important mechanical properties in polymers [18–20]. This behavior in hydrogels has a great role in its performance [21–24].
In this research nanocomposite hydrogels based on N-isopropylacrylamide and (3-acrylamidopropyl)trimethylammonium chloride were synthesized and their viscoelastic properties have been investigated at different temperatures. Since the vibration rheology of hydrogels is often complicated due to the slippage of the samples, we tried to characterize the gels behavior by compression tests. By introducing a hydrophobic anion, nanoclay and cationic co-monomer, the thermal response and viscoelastic properties of the hydrogels can be improved over a range of temperatures from below the lower critical solution temperature (LCST) to above LCST.
EXPERIMENTAL
Materials
N-Isopropylacrylamide (NIPAM) from Acros Organics Company was recrystallized with hexane. (3-acrylamidopropyl)trimethylammonium chloride (AMPTMA) from Aldrich Company was precipitated and washed with acetone and dried in vacuum. Water for preparation of polymer solutions was purified with ultrapure water purification system (ELGA lab Water). Bis(trifluoromethane)sulfonamide lithium salt (LiNTF2) (Aldrich, 99%) and NaCl (Fluka, 99%) were used as received. A water soluble initiator, 2,2'-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride as white crystalline powder with commercial name of VA-044 and tetramethylethylenediamine (TMEDA) as catalyst of polymerization were obtained from Sigma-Aldrich Company. Synthetic hectoritelaponite XLG was obtained from Rockwood Company with bulk density 1000 kg/m3 and surface area 370 m2/gr. N,N '-methylenbisacrylamide (Sigma-Aldrich Company) was used as received.
Synthesis of Nanocomposite Hydrogels
Water (1 mL), NIPAM (250 mg), AMPTMA (42.8 mg), N,N '-methylenbisacrylamide (1.5–1.9 mg), VA-044 (7.2 mg), TMEDA (0.04 mL) and laponite XLG (25.4 mg) were poured into a glass reactor. This mixture was ultra-sonicated for 10 min, stirred for 30 min and bubbled with nitrogen for 10 min before free radical polymerization at ice-bath temperature. Final product is placed into dialysis tube and dialysis is continued for 3–5 days.
Measurements
Calorimetric measurements were performed using a differential scanning calorimeter DSC Q2000, TA instruments in nitrogen atmosphere with a flow rate of 50 mL/min. The temperature was calibrated with mercury, D2O and indium. Prior to the heating scan, swollen hydrogels were cut into small fragments of mass of 20‒30 mg and were put in hermetically sealed aluminum pans at 0°C for 1 h. The measurements were performed by heating the samples from 0 to 60°C with heating rate of 1 grad/min. The characteristics volume phase transition temperature was determined from the DSC thermograms.
Rheological measurements were performed using a stress-controlled rheometer AR2000, TA instruments with a stainless steel 20 mm plate-plate measuring head. Flat gel samples with diameters 30–40 mm at 20°C were immersed in distilled water to avoid drying or syneresis of the gel upon compression and to avoid sample shrinking below the diameter of the plate upon heating. All samples were tested at constant temperature 20°C (which is below the LCST of hydrogels) and constant frequency of 1.0 Hz, while the shear stress was ramped from low to high until the hydrogels went beyond the elastic stress range and yielded. Oscillatory and compression measurements were made at temperatures from 20 to 45°C. Before the actual measurements, the samples were equilibrated for 1 h at each temperature. Frequency sweeps were then made using strains within the linear viscoelastic regime (1% strain). For the oscillatory measurements the samples were compressed to 0.01 N normal forces to ensure proper contact with the sample and the plate. Then, for the compression measurements, the plate was lowered at a speed on 30 μm/sec and normal force recorded. The compressive strain as well as normal stress was then calculated from the distance measurement and normal force.
Swelling experiments of the hydrogels were made gravimetrically in the temperature range from 20 to 45°C after the sample surfaces had been wiped with paper to remove excess water. The hydrogel samples were allowed to swell in deionized water for at least 1 day at each temperature (mass of the swollen gel WS). The dry weight of the hydrogels WD was measured after drying the gels under vacuum for 24 h.
Swelling behavior was expressed based on the water weight adsorbed by the gels and calculated by Eq. (1):
RESULTS AND DISCUSSION
The monomer, NIPAM, and comonomer, AMPTMA, with molar ratio of 11 : 1 (NIPAM : AMPTMA) were polymerized in deionized water in the presence of an initiator, catalyst, the cross-linker and hectoritelaponite XLG at 0°C.
The resulting hydrogels showed different characteristics depending on factors such as the amount of nanoclay, the type of salt, and co-monomer. The feed compositions of the samples chosen for different tests are shown in Table 1.
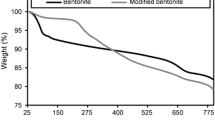
Swelling ratios of the hydrogels versus temperature is shown in Fig. 1. The swelling ratio at 20°C was highest for the cationic hydrogel. The swelling ratio of the sample S3 (nanoclay content 10%) was 48. The swelling ratio of the sample S2 in 5 mM LiNTF2 from 40 at 20°C decreased to 1 at 45°C. As the temperature increased, the swelling ratios of the PNIPAM and cationic hydrogels in 5 mM LiNTF2 decreased. When the temperature was above the LCST, a phase transition happened, and these hydrogels shrank in volume. The change in swelling ratio as a function of temperature variation around the LCST increased from PNIPAM to cationic hydrogels in 5 mM LiNTF2, indicating that the temperature sensitivity of the hydrogels synthesized in the presence of a cationic monomer and 5 mM NTF2 hydrophobic counter ion was improved. This novel PNIPAM-based system shows an interesting field to study Hofmeister effects. This work shows that the relative position of the ions in the Hofmeister series can be altered as a function of the degree of hydrophobic/hydrophilic character of the surfaces. The Hofmeister effects depend strongly on the hydration degree of the interfaces and the ions interacting with them.
Table 2 represents the DSC results of samples at temperature range from 0 to 60°C with heating rate of 1 grad/min. Analysis of the thermograms of the samples gives not only the onset temperature of the thermal collapse of the gels but also the enthalpy change associated with it. ΔH is related to the fraction of NIPAM units or segments which are free to undergo the deswelling process. Table 2 collects the data obtained from the analysis of DSC results.
DSC results confirm that at the presence of AMPTMA in PNIPAM network no thermal transitions are observed in distilled water (sample S2 and S3). But in the samples S1 and S2 swollen in 5 mM salt solution of LiNTF2, we can observe volume phase transition temperatures, around 35 and 39°C upon heating, respectively. The same behavior is observed in the swelling studies. The transition point of this sample depends on the concentration of NTF2 and composition of AMPTMA. The pure PNIPAM gel (S1) has the maximum heat flow at 31.8°C. The enthalpy of phase transition was calculated from the area of the endothermic peak and it is about 6.36 KJ/mol for PNIPAM hydrogel. The transition enthalpy ΔH decreases significantly when the hydrogels soaked in salt solution of LiNTF2, as was expected for systems where the conformational freedom of the PNIPAM chains decreases.
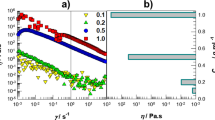
The linear-viscoelastic limit was determined with respect to strain. The elastic modules were determined from 0.1 to 10% strain for each sample. The recommended test is to check for the linear viscoelastic region limit by performing a strain sweep on the hydrogels. The hydrogel was allowed to reach equilibrium at each temperature for the appropriate amount of time. A strain sweep from 0.1 to 10% strain was then conducted at a frequency of 1.0 Hz on the hydrogel. The results of the strain sweeps for the hydrogels studied here are shown in Fig. 2. The hydrogels showed linear behavior (constant values as strain is varied) of elastic module up to 2% strain, and a strain of 2% was selected for subsequent sweeps. Above 2% strain the elastic module of the hydrogels then gradually decreased, indicating most likely slippage of the plate.
According to the strain sweeps, for frequency sweeps a new hydrogel was placed on the rheometer plate and the appropriate amount of time elapsed for the gel to reach equilibrium. Frequency sweeps from 0.1 to 100 rad/s were conducted at the linear viscoelastic region strain amplitude determined in the preceding section. The results of frequency sweeps are shown in Fig. 3. It can be seen that the elastic and loss module of all hydrogels followed a similar trend. At all frequencies, elastic module is higher than the loss module. As expected, the presence of PAMPTMA in the PNIPAM network (S2) significantly increased the module. The elastic modules of cationic hydrogels network in water was around 30 times greater than that of the homopolymer gel, showing the positive effect of cationic co-monomer on the elastic properties of the hydrogels. However, PNIPAM swollen in 5 mM LiNTF2 had the elastic properties significantly higher than PNIPAM, showing the effect of anionic NTF2 group on viscoelastic properties of the hydrogels. This shows that NTF2 binds to the cationic groups strongly enough to act as an additional cross-linking agent.
Figure 4 shows the prepared cationic hydrogels in swollen states at 20°C.
Oscillatory rheology experiments were performed to study the effects of various salts on the PNIPAM-based hydrogels. Figure 5 shows the variations of the storage modules G ′ with temperature at frequency of 30 rad/s for homo-PNIPAM hydrogels in the presence of different amounts of NaCl and LiNTF2 salt concentrations. As has been shown in Fig. 5, NaCl solution has a negative effect on elasticity of the hydrogels, because the elastic modules of homo-PNIPAM in 250 mM NaCl is less than that in homo-PNIPAM in water. The highest elasticity can be seen in homo-PNIPAM in mixture of 250 mM NaCl‒15 mM LiNTF2. Anyway, LiNTF2 solution has a positive effect on the elastic properties of the hydrogels. With higher concentration of LiNTF2, higher elasticity of the hydrogels was observed especially at temperatures above the LCST.
CONCLUSIONS
Based on free radical polymerization a series of PNIPAM based hydrogels have been prepared. Non-ionic and cationic hydrogels were prepared at different conditions. The motivation for the addition of cationic repeating units to the polymer was based on increasing the water sorption of the polymer. By introducing a hydrophobic anion, cationic co-monomer, and nanoclay one should be able to improve the rheological properties, thermal and swelling ratio of the hybrid hydrogels. However, the combination of a cationic monomer, a hydrophobic counter ion and nanoclay help to prepare stronger gels. This study outlines a protocol to assess the linear viscoelastic mechanical properties of hydrogels using oscillatory shear. The rheological properties of the PNIPAM hydrogels were in the order of PNIPAM in mixture of 15 mM LiNTF2 and 250 mM NaCl > PNIPAM in 15 mM LiNTF2 > PNIPAM in 5 mM LiNTF2 > PNIPAM in water > PNIPAM in 250 mM NaCl. And also the viscoelastic properties of cationic hydrogels were in the order of cationic hydrogels in water > cationic hydrogels–Nanoclay in water > cationic hydrogels–Nanoclay in 5 mM LiNTF2 > cationic hydrogels in mixture of 250 mM NaCl and 15 mM LiNTF2. It can be concluded that the mixture of NaCl and LiNTF2 salt solutions had a positive effect on storage modules of PNIPAM hydrogels. The overall conclusion is that strong thermally responsive PNIPAM gels may be obtained by adding a suitable amount of nanoclay additive to the reaction mixture or by using ionic interactions between the polymer and added salt.
REFERENCES
E. M. Ahmed, J. Adv. Res. 6, 105 (2015).
W. Abobatta, Adv. Agric. Environ. Sci. 1, 59 (2018).
F. F. Montesano, A. Parente, P. Santamaria, A. Sannino, and F. Serio, Agric. Agric. Sci. Procedia 4, 451 (2015).
M. F. Akhtar, M. Hanif, and N. M. Ranjha, Saudi Pharm. J. 24, 554 (2016).
Q. Chai, Y. Jiao, and X. Yu, Gels 3,1 (2017).
A. M. Mathur, S. K. Moorjani, and A. B. Scranton, J. Macromol. Sci., Part C 36, 405 (1996).
J. Liu and Y. Yin, Polym Sci. 1, 1 (2015).
D. S. Peterson, “pH-Sensitive Hydrogel,” in Encyclopedia of Microfluidics and Nanofluidics, Ed. by D. Li (Springer, Boston, MA, 2014).
N. Baıt, B. Grassl, C. Derail, and A. Benaboura, Soft Matter 7, 2025 (2011).
P. Zarrintaj, M. Jouyandeh, M. R. Ganjali, B. Shirkavand Hadavand, M. Mozafari, S. S. Sheiko, M. Vatankhah-Varnoosfaderani, T. J. Gutiérrez, and M. R. Saeb, Eur. Polym. J. 117, 402 (2019).
A. Usta and R. Asmatulu, J. Pharm. Drug Delivery Res. 5, 2 (2016).
M. Z. Urošević, L. B. Nikolić, S. S. Ilić-Stojanović, V. D. Nikolić, S. M. Petrović, and A. S. Zdravković, Adv.Technol. 7, 79 (2018).
A. Okudan and A. Altay, Int. J. Polym. Sci. 2019, 7324181 (2019).
S. A. Jadhav, V. Brunella, S. Sapino, B. Caprarelli, C. Riedo, D. Chirio, and M. Gallarate, J. Colloid Interface Sci. 541, 454 (2019).
Y. Chen, K. Zheng, L. Niu, Y. Zhang, Y. Liu, C. Wang, and F. Chu, Int. J. Biol. Macromol. 128, 414 (2019).
S. Rafieian, H. Mirzadeh, H. Mahdavi, and M. E. Masoumi, Sci. Eng. Compos. Mater. 2019, 154 (2019).
Z. Liu, Y. Faraj, X. J. Ju, W. Wang, R. Xie, and L. Y. Chu, J. Polym. Sci., Part B: Polym. Phys. 56, 1306 (2018).
B. Shirkavand Hadavand, F. Najafi, M. R. Saeb, and A. Malekian, High Perform. Polym. 29, 651 (2017).
A. Madhi and B. Shirkavand Hadavand, J. Compos. Mater. 54, 101 (2020).
B. Shirkavand Hadavand and H. Hosseini, Sci. Eng. Compos. Mater. 24, 691 (2017).
E. E. Charrier, K. Pogoda, R. G. Wells, and P. A. Janmey, Nat. Commun. 9,1 (2018).
G. Mattei, L. Cacopardo, and A. Ahluwalia, Materials 10,889 (2017).
R. Kocen, M. Gasik, A. Gantar, and S. Novak, Biomed. Mater. 12,025004 (2017).
L. Cacopardo, N. Guazzelli, R. Nossa, G. Mattei, and A. Ahluwalia, J. Mech. Behav. Biomed. Mater. 89, 162 (2019).
ACKNOWLEDGMENTS
This study was supported by the Laboratory of Polymer Chemistry of University of Helsinki and Department of Chemical Engineering, Abadan Branch, Islamic Azad University in Iran. The authors are grateful for the support. We are particularly grateful for the scientific assistance given by Prof. Heikki Tenhu at the Laboratory of Polymer Chemistry of University of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hosseini, H., Shirkavand Hadavand, B. Synthesis and Viscoelastic Properties of Smart Hydrogel. Polym. Sci. Ser. B 62, 394–399 (2020). https://doi.org/10.1134/S1560090420040053
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090420040053