Abstract
Using the method of electrochemical anodization, aluminum oxide porous films are obtained in a sulfuric acid solution. The morphology of the aluminum oxide surface is studied by the method of scanning electron microscopy. The high-quality elemental analysis of the initial and oxidized Al films is performed using the method of electron microprobe analysis. A protective composite polypyrrole-aluminum oxide film is produced on the aluminum surface on top of a porous aluminum oxide film in the galvanostatic oxidation mode by the electrochemical synthesis method. The properties of the polypyrrole–aluminum oxide composite film are studied by the methods of voltammetry, as well as impedance and FTIR spectroscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
According to the data [1], aluminum (Al) waste due to corrosion amounts to 2–3% every year; therefore, the protection of Al and aluminum alloys from corrosion is an important industrial problem.
Aluminum is considered to be a metal that is quite resistant to general corrosion. In some cases, the resistance of Al to corrosion is provided by the natural 5–100 Å oxide film formed on its surface [2]. Such layers are not particularly effective in protecting the metal against pitting corrosion that can affect Al in the chloride-ion media [3]. During pitting corrosion, the process develops locally [4–6]. The transfer of electrons between regions with a different electrochemical potential, which can make up a large number on the Al-base alloy, leads to hazardous, deep fractures almost through the entire depth of the metal. To improve the resistance of the metal to corrosion, its surface undergoes treatment. In this case, the Al-base alloys are most often oxidized by special compositions based on the compounds of hexavalent chromium (Cr6+). However, these substances are very poisonous; therefore, the researchers are actively looking for a substitute for them.
Among the electrically conductive organic polymers, we can identify polymers based on five-membered heterocycles with polypyrrole (PP) as a typical representative (Fig. 1).
When coated on the surface, conductive polymers such as PP can protect Al alloys against corrosion quite well. A necessary condition for this is the correct selection of an electron transfer mediator used in coating a conductive polymer [7, 8].
In 1979, A.F. Diaz synthesized PP (Fig. 1) using electrochemical rather than chemical polymerization [9]. A film of a doped polyconjugated polymer (PCP) was obtained on an electrode by electrochemical machining, which consists in the anode oxidation of a pyrrole monomer. This electrochemical method has an important advantage: it allows obtaining thin films with a controlled thickness, which is interesting in terms of using such polymers in electronics and optoelectronics [10].
The use of PCP polymers in composite coatings is one of the new directions in protecting metals from corrosion. The correctly selected PCP compositions exert a pronounced anticorrosion action due to a number of reasons [11], including the oxidizing-reducing properties of PCP, which assigns the potential of the metal surface outside the zone of active dissolution. The anticorrosive action is determined by the reactions of polymer doping leading to anion binding [12]. In addition, the conventional function of polymer coatings as a mechanical blocker of active substances has been retained. The PCP deposition cannot be observed on the Al electrode surface due to the presence of the oxide layer on the surface.
The purpose of this work is to synthesize and to study a PP–aluminum oxide nanocomposite protective film on the Al surface.
EXPERIMENTAL
The experiment conventionally consisted of two stages.
At the first stage, the technology of coating a highly developed porous structure of Al oxide on the aluminum plate surface was developed. The scientific research on clarifying the mechanism of corrosive and electrochemical reactions on Al-electrodes in aqueous media is performed using direct and alternate current methods [13–18].
Al2O3 was produced from an aluminum foil (purity of 99.99%) plate of 3 × 3 cm2, which was 100 µm thick. The Al plate anode was immersed with a holder in a 500-cm3 cell, which in addition to an electrolyte represented by an aqueous solution of sulfuric acid contained a counter electrode made of a stainless-steel plate of 3 × 3 cm2, which was 0.5 mm thick. The potentials were measured relative to the standard calomel electrode (SCE).
The samples were prepared in two stages. At the first stage, the aluminum foil was anodized by applying an armor oxide layer on the electrolyte without a pyrrole monomer.
At the second stage, a solution with a pyrrole monomer and sodium sulfate was used.
PP was obtained on the Al plate by the galvanostatic method at the current density of 1 mА/cm2.
A Princeton Applied Research 273 A potentiostat-galvaostat was used as a device maintaining the electrochemical parameters. Electropolymerization was performed at a constant current with the density of 1 mА/cm2. The circuit was disconnected after the necessary electricity had passed.
Figure 2 presents microimages of the surfaces of the fractures and front surfaces of the Al oxide films obtained on the alternate (upper row) and direct (bottom row) currents on the Al surface. The microimages were made by a Merlin scanning microscope (Karl Zeiss, Germany).
Figures 1 and 2 show that the Al oxide film obtained on alternate current has a more porous structure (with a pore size of 5–20 nm) than the film obtained on a direct current. We may assume that it should be difficult for PP to penetrate the Al surface through such porous structures.
In contrast, the structure of Al oxide obtained on an alternating current has a looser structure, contains many voids, and the volume of Al oxide is much less compared to the void space. In our opinion, this structure better acts as an armor component for the future nanocomposite and it was the only one further considered.
Figures 3a and 3b show microimages of the initial and Al-oxide-modified aluminum plate surfaces, respectively.
As shown in Fig. 3a, the initial surface of the aluminum plate contains pronounced and mutually parallel mill pass lines having a periodic character.
Figure 3b shows that the oxidized surface of the aluminum plate has a pronounced porous character with a pore size of 100 to 1000 nm.
The upper layer in these images (the lighter region) refers to Al oxide and has a thickness of ∼2.5 µm, the other part of the plate, referring to the initial aluminum, is found beneath this region (the darker region). Figure 4 displays the spectra of the elemental analysis of the distinguished regions in the initial Al (region, spectrum 1) and the oxidized film (region, spectrum 2). No inclusions of other phases were detected in the distinguished regions related to the initial aluminum. The distinguished region that is related to the oxide film was found to contain a large amount of oxygen corresponding to the chemical formula Al2O3, and microscopic amounts of sulfur that remained on the oxide film surface due to the action of the sulfur-containing electrolyte.
At the second stage, we studied the technology of coating PCP on top of the highly developed porous structure of Al oxide, which covers the aluminum plate surface, by the method of electrochemical synthesis in the galvanostatic oxidation mode. The experimental structures were coated and their electrochysical characteristics were studied.
The infrared spectra were collected on a Magna-IR 850Micollet spectrophotometer.
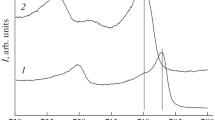
Figure 5 shows the results of the FTIR spectroscopy [19] that PP films were subjected upon the reflection from the surfaces of the initial (the upper plot) Al plate and oxidized (the lower plot) Al plate.
Both spectra showed the bands of valence vibrations that are PP typical and were recorded in [20–27]. The peaks at 772, 811, and 920 cm–1 can be related to the C–H vibrations, the peaks typical at 1558 and 1487 cm–1 correspond to the С=С vibrations, while the peaks at 1686 and 1315 cm–1 represent the C=N and C–N vibrations, respectively. The small peaks at 3522 cm–1 indicate the presence of the N–H vibrations.
In general, the IR spectroscopy data show that both Al-base electrodes are coated with a PP-based stable protective film.
Figures 6a and 6b show the microimages of the surface of PP deposited on the surface with the initial Al and Al oxide, respectively.
It is clear that the polymer structure strongly depends on the surface composition. It has a looser structure on the Al surface but is more uniform and denser on the porous Al oxide surface.
DISCUSSION
Figure 7 illustrates the voltamperograms of the galvanostatic synthesis of the PP film on the surface of the initial (the lower plot) and Al2O3-coated Al plates. The synthesis on the oxide surface occurs with excessive overpotential due to the high impedance of the oxide film.
Figure 8 plots the dependences for the impedance log of the sample at a frequency of 0.1 Hz on the time of holding in the dilute Harrison solution. The impedance measurements were taken with a Gamry PC-4 impedance meter under the procedure described in [28]. During the entire observation time, the samples having Al2O3 demonstrated an impedance higher by an order of magnitude (the upper plot) than the Al surface free of oxide (the lower plot). Thus, we may conclude that the high impedance of the oxidized samples is determined by the presence of oxide.
The oxidizing and reducing properties of the surface were studied by the potentiodynamic scanning of the surface under the standard procedure described, e.g., in [29]. Figure 9 presents the Tafel plots reflecting the redox processes at the boundaries with the solution of the initial plate, the PP-coated plate, and the plate coated with an oxide and PP.
According to the obtained experimental data, anodization of the Al surface and the subsequent coating of PP under the given procedure of coating increase the electrochemical potential of the surface by ~ 0.8 V, which can significantly slow down the corrosive processes on this surface.
Thus, the oxide coating increases the surface impedance and the PP coating improves its electrochemical potential. An oxide also contributes to better adhesion of the PP to the surface.
CONCLUSIONS
The IR spectroscopy data show that both Al-base electrodes are coated with a PP-based stable protective film, which is proved by the occurrence of the typical peaks of the sought material. The impedance measurements showed that the presence of an oxide together with PCP on the Al surface greatly improves the barrier properties of the surface, which manifests itself in increased impedance. The potentiodynamic measurements showed that, together with an oxide, PP significantly increases the electrochemical potential of the surface, making it more “noble”, which improves its resistance to corrosion.
REFERENCES
Jurczak, W., Study of the corrosion resistance of ship aluminum alloys, Sci. J. Polish Naval Acad., 2016, vol. 3, no. 206, pp. 37–65.
Sinyavskii, V.S., Val’kov, V.D., and Kaminin, V.D., Korroziya i zashchita alyuminievykh splavov (Corrosion and Protection of Aluminum Alloys), Moscow: Metallurgiya, 1996.
Karimova, S.A., Zhilkov, V.P., Mikhailov, A.A., Chesnokov, D.V., Igonin, T.N., and Karpov, V.A., Natural-accelerated testing of aluminum alloys under the influence of the marine atmosphere, Korroz.: Mater., Zashch., 2012, no. 10, pp. 1–3.
Tallman, D.E., Pae, Y., and Bierwagen, G.P., Conducting polymers and corrosion: Part 2 - polyaniline on aluminum alloys, Corrosion, 2000, vol. 56, p. 401.
Olive, J.M., Mechanistic model of corrosion pitting on 2043 T3 alloy, Solidif. Met. Alloys, 1996, no. 28, pp. 226–232.
Frantziskonis, G.N., Simon, L.B., Woo, J., and Matikas, Th.E., Multiscale characterization of pitting corrosion and application to an aluminum alloy, Eur. J. Mech. A: Solids, 2000, vol. 19, pp. 309–318.
Tallman, D.E., Spinks, G., Dominis, A., and Wallace, G.G., Electroactive conducting polymers for corrosion control, Part 1. General introduction and a review of nonferrous metals, J. Solid State Electrochem., 2002, vol. 6, no. 2, pp. 73–84.
Levine, K.L., Tallman, D.E., and Bierwagen, G.P., The mediated electrodeposition of polypyrrole on aluminium alloy, Austral. J. Chem., 2005, vol. 58, no. 4, pp. 294–301.
Diaz, A.F., Kanazawa, K.K., and Gardini, G.P., Chemical communications, electrochemical polymerization of pyrrole, J. Chem. Soc., Chem. Commun., 1979, pp. 635–636.
Skotheim, T.A. and Reynolds, J.R., Handbook of Conducting Polymers, Boca Raton, FL: CRC, 2007.
Tallman, D.E., Vang, C., Wallace, G.G., and Bierwagen, G.P., Direct electrodeposition of polypyrrole on aluminum and aluminum alloy by electron transfer mediation, J. Electrochem. Soc., 2002, vol. 149, no. 3, pp. C173–C179.
Kendig, M., Hon, M., and Warren, L., Smart corropsion inhibiting coatings, Prog. Org. Coat., 2003, vol. 47, pp. 183–189.
Pol’shina, E.Yu. and Shavkunov, S.P., Corrosion and electrochemical behavior of aluminum in sulfuric acid, Polzunov. Vestn., 2008, no. 3, pp. 181–185.
Averin, I.A. and Gubich, I.A., Analysis of models for the formation and ordering of the porous structure of aluminum oxide, Tekh. Nauki, Mashinostr, Mashinoved., 2003, no. 2 (26), pp. 91–100.
Lee, W., Schwirn, K., Steinhart, M., Pippel, E., Scholz, R., and Gösele, U., Structural engineering of nanoporous anodic aluminium oxide by pulse anodization of aluminium, Nat. Nanotechnol., 2008, vol. 3, pp. 234–239.
Jessensky, O., Müller, F., and Gösele, U., Self-organized formation of hexagonal pore arrays in anodic alumina, Appl. Phys. Lett., 1998, vol. 72, no. 10, pp. 1173–1175.
Markaryan, E.S., Kinetics of electrochemical oxidation of aluminum in sulfur electrolyte, in Fiziko-khimicheskie aspekty izucheniya klasterov, nanostruktur i nanomaterialov: mezhvuz. sb. nauch. tr. (Physico-Chemical Aspects of the Study of Clusters, Nanostructures and Nanomaterials, Collection of Articles), Samsonov, V.M. and Sdobnyakov, N.Yu., Eds., Tver: Tver. Gos. Univ., 2013, no. 5, pp. 176–179.
Levine, K.L., Tallman, D.E., and Bierwagen, G.P., Mott-Schottky analysis of aluminum oxide formed in the presence of different mediators on the surface of aluminium alloy 2024-T3, J. Mater. Process. Technol., 2008, vol. 199, pp. 321–326.
Malev, V.V., Kondrat’ev, V.V., and Timonov, A.M., Polimer-modifitsirovannye elektrody: monografiya (Polymer Modified Electrodes), St. Petersburg: Nestor-Istoriya, 2012.
Chougule, M.A., Pawar, S.G., Godse, P.R., Mulik, R.N., Sen, S., and Patil, V.B., Synthesis and characterization of polypyrrole (PPy) thin films, Soft Nanosci. Lett., 2011, vol. 1, pp. 6–10.
Kharat, H.J., Kakade, K.P., Savale, P.A., Dutta, K., Ghosh, P., and Shirsat, M.D., Synthesis of polypyrrole films for the development of ammonia sensor, Polym. Adv. Technol., 2007, vol. 18, no. 5, pp. 397–402.
Tian, B. and Zerbi, G., Lattice-dynamics and vibrational-spectra of polypyrrole, J. Chem. Phys., 2009, vol. 92, no. 6, pp. 3886–3891.
Arora, K., Chaubey, A., Singhal, R., Singh, R.P., Pandey, M.K., Samanta, S.B., Malhotra, B.D., and Chand, S., Application of electrochemically prepared polypyrrole-polyvinyl sulphonate films to DNA biosensor, Biosens. Bioelectron., 2006, vol. 21, no. 9, pp. 1777–1783.
Liu, A.S., Bezerra, M.C., and Cho, L.Y., Electrodeposition of polypyrrole films on aluminum surfaces from a p-toluene sulfonic acid medium, Mater. Res., 2009, vol. 12, no. 4, pp. 503–507.
Liua, A.S. and Oliveira, M.A.S., Electrodeposition of polypyrrole films on aluminum from tartrate aqueous solution, J. Braz. Chem. Soc., 2007, vol. 18, no. 1, pp. 143–152.
Trung, V.Q., Tung, D.N., and Huyen, D.N., Polypyrrole/Al2O3 nanocomposites: Preparation, characterization and electromagnetic shielding properties, J. Exp. Nanosci., 2009, vol. 4, no. 3, pp. 213–219.
Scienza, L.C. and Thompson, G.E., Preparation and surface analysis of PY/SDBS films on aluminum substrates, Polimer.: Ci. Tecnol., 2001, vol. 11, no. 3, pp. 142–148.
Loveday, D., Peterson, P., and Rodgers, B., Fundamentals of electrochemical impedance spectroscopy, J. Coat. Technol., 2004, no. 8, pp. 46–52.
Mansfeld, F. and Bertocci, U., Electrochemical Corrosion Testing, SPT 727, West Conshohocken, PA: Am. Soc. Test. Mater., 1979.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 17-03-000121 А.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by L. Mukhortova
Rights and permissions
About this article
Cite this article
Tomaev, V.V., Levin, K.L., Stoyanova, T.V. et al. Synthesis and Study of a Polypyrrole–Aluminum Oxide Nanocomposite Film on an Aluminum Surface. Glass Phys Chem 45, 291–297 (2019). https://doi.org/10.1134/S1087659619040126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659619040126