Abstract
The results of studies of the influence of dust from electrostatic precipitators of cement kilns on the synthesis of a binder containing mainly dicalcium silicate are demonstrated. The presence of alkali potassium and sodium oxides, as well as sulfate ions in the dust of electrostatic precipitators during annealing of a carbonate-siliceous mixture, promotes the formation of a solid solution based on the structure of β-modification 2СаО‧SiO2, an increase the defectiveness of its crystal lattice, maintaining a metastable state during cooling and a rise in activity under hydrothermal conditions of hardening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In the production of binders, emissions of fine particles, dust, occurs. The basis of the cement manufacturing process is annealing of Portland cement clinker at temperatures of 1350–1450°C. By phase composition, Portland cement clinker is mainly represented by calcium silicates: up to 60% of tricalcium 3СаО·SiO2 (alite C3S) and 20–26% of dicalcium 2CaO·SiO2 (belite C2S) silicates. Alite and belite are formed in a cement kiln under various thermal conditions and differ in hydraulic properties. The formation of С3S (the temperature of formation is 1400–1450°С) is the most power-consuming; the belite phase is formed at 1200–1300°С. Alite is the most active in hydraulic properties. It quickly hydrates and gives maximum strength to cement stone. Belite is characterized by slow setting and a gaining strength under natural conditions, but in the later stages of hardening it intensively gains strength and can exceed the strength of hydrated alite. So, after 3 days of hardening, the degree of belite hydration is more than 3 times lower than that of alite; after 6 months, the belite phase hydrates by 56%, which is 78.6% of the degree of alite hydration [1, 2].
Dicalcium silicate (2СаО·SiO2) melts congruently at 2130°C, and is characterized by a very complex polymorphism. In the range of 20–1500°С, there are six crystalline forms of 2СаО·SiO2: α, α'Н, α'L, βH, βL, and γ, the stability interval of which is different during heating and cooling. When annealing Portland cement clinker, a β-C2S transition to a stable γ-C2S modification is possible, which in vivo does not have binder properties.
Since the hydraulic activity of various modifications of dicalcium silicate varies significantly, the problem of stabilization of β-2СаО·SiO2 is relevant for the production of belite binders and Portland cement clinker. Various factors influence the stabilization process: conditions for the formation and growth of nuclei, particle size, cooling rate, temperature, and the presence of impurities that can isomorphically replace Са2+ or Si4+. The isomorphic capacity of the crystal lattice of dicalcium silicate can be up to 6 wt %; isomorphic substitution is possible in both the cationic and anionic parts of the structure. Heterovalent isomorphism of the type Si4+ Si+ + Si3+ is the most common [3].
In the production of Portland cement clinker, the exhaust gases pass through several stages of purification, the collected dust is a waste product. According to the steps of gas purification in electrostatic precipitators, dust differs in chemical composition. At the first stages, the dust is close in composition to the raw materials, at the last stage it is characterized by a high content of alkaline and sulfate compounds. The dust of the 4th purification stage was used in the study. According to their chemical composition, dust of electrostatic precipitators contains, in addition to CaO and SiO2, oxides Al2O3, Fe2O3, MgO, SO3, R2O, and others, the presence of which can affect the stabilization process of one or another modification of 2СаО·SiO2 [4, 5].
The aim of the work is to study the effect of dust from electrostatic precipitators of cement rotary kilns on the synthesis and stabilization of dicalcium silicate mainly in the form of β-modification and to study the process of hardening of a binder based on it under autoclaving conditions.
EXPERIMENTAL
In the work, we synthesized dicalcium silicate in the form of γ- and β-modifications in the presence of dust from electrostatic precipitators of cement kilns from chemically pure CaCO3 and SiO2, as well as from natural carbonate and clay components. The phase composition was determined using chemical methods (by the number of bound oxides of the mixture) and X-ray phase analysis (by the intensity of diffraction reflexes of crystals with decoding of the interplanar spacings of a specific phase) by the ARL9900 Intellipower Workstation X-ray diffractometer [6]. Data was processed with software systems Difwin, Crystallographica Search-Match. Hydraulic activity under hydrothermal conditions (saturated water vapor pressure 0. MPa; temperature 175°C; 2 h temperature and pressure rise, keeping at constant temperature and pressure, 2 h temperature and pressure reducing) were evaluated by compressive strength of cylinder samples d = 20 mm, made by semi-dry pressing at a pressure of 20 MPa. Samples were prepared in a mixture with quartz sand. The amount of sand introduced was calculated taking into account the CaO content in the free state in the annealed product. The amount of water for mixing was calculated taking into account the flow rate for hydration of free calcium oxide, evaporation during quenching and molding moisture of the mixture (W = 6.5%).
When calculating the ratio of the initial components of the mixtures for the synthesis of dicalcium silicate in the form of γ- and β-modifications, the content of calcium oxide in the free state in the annealing product was simultaneously accounted for. The prepared mixtures were annealed in a silica furnace at temperatures of 1000, 1050, and 1100°C for 40 min. The annealed product was ground to a specific surface area of 400 m2 kg–1. Dust of electrostatic precipitators was added to the mixture in an amount of 5 wt %. Data on the chemical composition of the materials used, the composition of the mixtures and annealing products are given in Tables 1, 2, the phase composition of the annealing products, in Table 3.
In the annealing product, the CaO content in the free state was determined by the ethyl glycerate method, and the SiO2 content by the photocolorimetric method [7] (Table 4). The mineral content of dicalcium silicate, aluminate, and calcium ferrite, as well as non-decomposed CaCO3, was calculated from the results of a chemical analysis of the annealing product (Table 5).
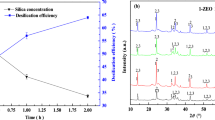
The phase composition of the annealing product is confirmed by the results of X-ray phase analysis (Figs. 1, 2). When annealing a mixture of CaCO3 and SiO2 in the temperature range of 1000–1100°С for 40 min, 20–25% of CaCO3 remains in the non-decomposed state (Table 4), the product contains oxides of CaO and SiO2 in the free state and dicalcium silicate in the form of β- and γ-modifications (Figs. 1a–1d, Å: 3.01, 2.788, 2.755, 2.614, 2.194).
It was found [8] that the smaller the β-2CаО·SiО2 crystals, the less likely the formation of γ-shape nuclei, in this case the β-modification is stabilized. An increase in the annealing temperature leads to a decrease in the content of CaCO3, oxides in the free state, and a rise in the amount of 2CаО·SiО2.
Crystallochemical stabilization is based on the formation of 2CаО·SiО2 solid solutions with some additives. Cations (anions) of Na+, K+, Mg2+, SO42– present in dust enter the 2CаО·SiО2 crystal lattice, which undergoes changes in this case, resulting in its stabilization. At the same time, additives can affect the reactivity of the compound. Stabilizing additives in some cases significantly increase, in others sharply reduce hydration activity. “Minor” impurity oxides (Na2O, K2O, MgO, SO3, etc.), which are always contained in the initial raw materials, create favorable conditions for the formation of phases with a complex of isomorphic substitutions in their lattices, while lowering the temperature of mineral synthesis. The crystal chemical features of these phases are well known; in relation to 2CаО·SiО2, solid solutions are called belites.
It was established [9] that the metastable β-modification of 2CаО·SiО2 is stabilized by the introduction of ions of different charge states into the structure during synthesis (heterovalent isomorphism). A prerequisite for the implementation of substitutions of this type is charge compensation. Isomorphic elements in the lattices of matrix minerals can occupy different positions. Thus, Na+, K+, Mg2+ ions in 2CаО·SiО2, as a rule, replace Ca2+ ions, and in [SiO4] tetrahedra there can be S6+ ions instead of Si4+. It was determined in [10] that the more pairs of heterovalent isomorphic substitutions present in the mineral structure, the greater the distortion of the structure. Due to the deformation of the crystal lattice, the properties of the minerals and materials containing them can be significantly changed (for example, hydration and hardening characteristics). The stabilization by a smaller amount of impurities may be explained by relatively small unit cell volume of β-2CаО·SiО2 (0.343 nm3) in comparison with the volumes of other high-temperature modifications of this compound (for example, in α'-2CаО·SiО2 1.08 nm3) [11]. The content of impurity ions in the β-2CаО·SiО2 crystal lattice, which can be incorporated into its structure and replace matrix ions, can be ~4–6 wt %.
The introduction of electrostatic precipitators into the dust mixture containing a significant amount of oxides R2O (R is an alkali metal), MgО, and SO3 leads to intensification of not only the process of decarbonization of calcium carbonate, but also the interaction of CaO with silica [12]. Dicalcium silicate (Figs. 2b–2d, Å: 2.788, 2.759, 2.618, 2.196) is contained only in the form of a β-modification; self-scattering characteristic of the transition to the γ-form does not occur. The presence of MgO, K2O, SO3 oxides leads to crystallochemical stabilization of the β-modification of 2СаО·SiO2, the diffraction maximum characteristic of the γ-modification of 3.01 Å is absent. The content of a significant amount of K2O (~25%) in the dust of electrostatic precipitators could provide crystallochemical stabilization of the β-modification of 2СаО·SiO2 without the participation of other oxides [13]. The shift of the diffraction deviations characteristic of the β modification towards large angles indicates the incorporation of potassium ions K+ instead of Ca2+, S6+ instead of Si4+ into the structure of dicalcium silicate, which is an X-ray sign of an increase in the unit cell parameters and is in good agreement with the dimensional characteristics of mutually substituting ions (rCa2+ = 1.04 Å, rK+ = 1.33 Å, rSi4+ = 0.39 Å, rS6+ = 0.29 Å). The difference in the sizes of the radii of the ions Ca2+ and K+ 21.8%, Si4+ and S6+ is 25.6%, as well as the content of significant amounts of K2O in dust of electrostatic precipitators in comparison with SO3 explains the shift of the diffraction maxima for β-2СаО·SiO2 towards large, not smaller, angles, which, as a whole, increases the defectiveness of the crystal structure of the β-2СаО·SiO2 phase and its hydration activity [14].
In the annealing product based on chalk and clay with the addition and without dust of electrostatic precipitators except β-2СаО·SiO2 (d, Å: 2.784, 2.637, 2.194), aluminate (d, Å: 2.988, 2.578), calcium ferrite 2СаО·Fe2O3 (d, Å: 2.79, 2.698), CaO in the free state (d, Å: 2.414, 1.702) (Figs. 2a, 2b) were found, which confirms the data on the calculation of the phase composition according to the results of chemical analysis. With the introduction of electrostatic precipitators into the dust mixture, an increase in the intensity of the diffraction maximum of 2.79 Å, characteristic of β-2СаО·SiO2, is observed on the X-ray diffraction patterns of the calcination product, which indicates a significantly higher content of belite mineral. Consequently, the use of dust from electrostatic precipitators of cement kilns makes it possible to produce a binder containing dicalcium silicate in the form of a β-modification at relatively low temperatures.
The results obtained are characteristic of belite clinkers, no production of which, given their low hydration activity, currently occurs. In the work, to activate the belite component of clinker, hydrothermal hardening was used at a temperature of 175°C, which is typical for lime-sand mixtures [15]. After autoclaving and drying, the samples were tested for compressive strength, the content of Ca(OH)2 in the free state was determined (Table 6), and the phase composition was evaluated by the results of X-ray phase analysis (Fig. 3).
Lime-quartz binder, traditionally used in the manufacture of autoclaved hardening products (silicate brick, cellular autoclave products), is characterized by a relatively low compressive strength of 10.86 MPa, while a significant amount of Ca(OH)2 remains in the free state. The binder containing the calcining product (CaCO3 + SiO2) after hydrothermal hardening is characterized by a significant increase in strength to 26.8 and 34.18 MPa at temperatures of 1050 and 1100°C. The binder with the addition of dust of electrostatic precipitators up to 51.0 MPa at a temperature of 1100°C has the highest strength parameters. The reason for this is the increased content of dicalcium silicate in the form of β-modification (up to 61.88%) (Table 5) as a result of accelerated hydration of β-2СаО·SiO2 at hydrothermal treatment (in comparison with normal hardening conditions) and the interaction of free calcium oxide with silica sand with the formation of calcium hydrosilicates.
Defective structure is a factor affecting the chemical properties of the β-2СаО·SiO2 mineral. Replacing a certain amount of Ca2+ ions with K+ and Na+ ions, as well as Si4+ with S6+, not only stabilizes the β-modification of dicalcium silicate, but also significantly increases its hydraulic activity [13]. The activity of a binder synthesized from natural raw materials using dust from electrostatic precipitators of cement kilns exceeds 60 MPa, and when hydrated, all calcium hydroxide is absorbed. The phase composition of the hydrated product is represented by calcium hydrosilicates, which is confirmed by the results of X-ray phase analysis (Figs. 3a, 3b). When using an annealing product based on chalk, clay with the addition of dust of electrostatic precipitators, the hydrated phase of the clinker component is represented by both low basic CSH (B) (d, Å: 3.043, 2.823), and highly basic C2SH (A) (d, Å: 4.22, 3.943, 3.555, 3.278) with calcium hydrosilicates (Fig. 3b). Hydration of β-2СаО·SiO2 occurs simultaneously with the hydration of calcium aluminates and ferrites (CaО·Al2O3 and 2СаО·Fe2O3) resulting in the formation of hydroaluminate (d, Å: 3.126, 2.30) and calcium hydroferrite (d, Å: 4.048, 2.069), which contribute to the hardening of the structure of the binder. Calcium hydroxide formed during CaO hydration is completely bound by silica to calcium hydrosilicates with a CaO/SiO2 ratio of ˂1.5, which increases the amount of low-basic high-strength calcium hydrosilicates of the CSH (B) type (partially crystallized tobermorite-like calcium hydrosilicate with a CaO : SiO2 ratio of 1.5 and variable amount of water) [14].
The use of a binder, containing predominantly β-2СаО·SiO2, can significantly increase the strength characteristics. The phase composition of the binder obtained from natural raw materials with the addition of 5 wt % dust of cement rotary kilns indicates the possibility of producing belite clinkers at relatively low annealing temperatures in comparison with the currently produced alite clinkers. The combined hydration of the belite phase and the interaction of the free calcium oxide of the calcination product with silica sand make it possible to increase the number of hydrated phases after hydrothermal hardening and to produce a high strength binder on this basis. The accelerated hydration of dicalcium silicate at elevated temperatures and pressures increases the hydration phases.
CONCLUSIONS
An intensification of the process of the belite phase formation in the β-modification at relatively low temperatures was theoretically justified and experimentally established as a result of the replacement of a certain amount of Ca2+ ions by K+ and Na+ ions, as well as Si4+ by S6+ contained in the dust of electrostatic precipitators of rotary cement kilns. An increase in the structural imperfection of β-2СаО·SiO2 dicalcium silicate in the presence of a complex of impurities significantly increases the hydraulic activity of this mineral under hydrothermal conditions. The strength of an autoclaved binder based on belite clinker is six times higher than the strength characteristics of a lime-sand binder under constant curing conditions as a result of accelerated hydration of the belite phase and the formation of a larger amount of hydrated phase such as high-strength low-basic calcium hydrosilicates.
REFERENCES
Tailor, H.F.W., Cement Chemistry, London: Teklford Thomas, 1997.
Gareev, R.R., Korolev, A.S., Shaimov, M.Kh., and Trofimov, B.Ya., Refractories & Industrial Ceramics, 2006, vol. 47, no. 6, pp. 381–385. https://doi.org/10.1007/s11148-007-0012-x
Wang, Y.G., Zou, B.S., and Kuo, K., H.X., J. Mater. Sci., 1989, vol. 24, pp. 877–880. https://doi.org/10.1007/BF01148771
Nurymbetov, B.Ch., Turemuratov, Sh.N., Zhukov, A.D., and Asamatdinov, M.O., Vestn. MGSU., 2017, vol. 12, no. 4(103), pp. 446–451. https://doi.org/10.22227/1997-0935.2017.4.446-451
Aleksandrov, A.V. and Nemchinova, N.V., Vestn. Irkutskogo Gos. Tekhn. Univ., 2016, vol. 20, no. 11, pp. 170–183. https://doi.org/10.21285/1814-3520-2016-11-170-183
Chizhov, P.S., Lit’e & Metallurgiya, 2011, vol. 60, no. 2, pp. 172–174.
Butt, Yu.M. and Timashev, V.V., Praktikum po khimicheskoi tekhnologii vyazhushchikh materialov (Workshop on the Chemical Technology of Binders), Moscow: Vysshaya Shkola, 1973.
Boikova, A.I., Khimiya silikatov i oksidov (Chemistry of Silicates and Oxides), Leningrad: Nauka, 1982.
Boikova, A.I., Tsement, 1982, no. 9, pp. 7–10.
Boikova, A.I., Tsement, 1992, no. 2, pp. 17–19.
Shmanina, E.A., Abstracts of Papers, Nauchnyi poisk. Tekhnicheskie nauki: Materialy tret’ei nauch. konf. aspirantov i doktorantov. Yuzh.-Ural. Gos. Univ. (Scientific Search. Engineering: Materials of the Third Sci. Conf. of Graduate Students and Doctoral Students. South Ural. State Univ.), Chelyabinsk: Izd. Tsentr YuUrGU, 2011.
Ermolenko, E.P. and Klassen, V.K., Sb. Dokl. “Alit-inform” (Coll. of Reports “Alit-inform”)2012, no. 3, pp. 44–53.
Gutt, W. and Osborn, E.F., Cement Technol., 1970, vol. 1, no. 4, pp. 121–125.
Bikbau, M.Ya., Tsement i ego primenenie (Cement and Its Application), 2006, no. 5, pp. 64–67.
Butt, Yu.M. and Rashkovich, L.N., Tverdenie vyazhushchikh pri povyshennykh temperaturakh (Binder Hardening at Elevated Temperatures), Moscow: Stroiizdat, 1965.
ACKNOWLEDGMENTS
The authors are grateful to the staff of the Center for High Technologies of Belgorod State Technological University named after V.G. Shukhov for determining the chemical composition of the raw materials used, as well as the Interdepartmental Laboratory for X-ray phase analysis of samples of binder synthesis and hydration products for analyses of the samples under study, special thanks to the Department of Cement Technology and Composite Materials for providing installations for determining the technological properties of binders.
Funding
This work was carried out as part of a development program for a reference university based on the Belgorod State Technological University Named after V.G. Shukhov, as well as in the framework of research no. 532-2018-NIR from 12.06.2018
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kudeyarova, N.P., Bushueva, N.P. & Panova, O.A. Synthesis of Dicalcium Silicate in the Presence of Dust from Electrostatic Precipitators of Cement Kilns. Russ J Appl Chem 93, 813–820 (2020). https://doi.org/10.1134/S1070427220060063
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220060063