Abstract
Bisazomethines derived from D,L-camphor, 2-adamantanone, and aliphatic diamines were prepared. The possibility of using bisazomethines as vulcanization accelerators was examined. The physicomechanical parameters of vulcanizates based on SKI-3 and their resistance to thermal oxidative aging were determined. The influence of the bisazomethine structure on the vul canization rate was found. Among the compounds synthesized, N,N′-(propane-1,3-diyl)bis(1,7,7-trimethylbicyclo[2.2.1]heptan-2-imine) shows the best set of vulcanization and operation properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aromatic azomethines (Schiff bases, imines, aldimines) are very important compounds for research and practice. They are used in medicine, perfumery, color photography, electronics, textile and rubber industries, and agriculture [1]. The presence of amino groups in azomethines makes it possible to use them as modifiers, including those for aluminosilicate microspheres. Treatment of the microsphere surface with such azomethines enhances the affinity of the microspheres for the polymer matrix and improves their distribution [1].
Previously, adamantane-containing azomethines were studied as rubber stock vulcanization accelerators [2].
In this study, we examined the possibility of using the synthesized bisazomethines containing terminal framework substituents (camphor and adamantyl groups) as rubber stock vulcanization accelerators. Introduction of such groups into bisazomethine molecules allows preparation of compounds exhibiting a wide spectrum of practically useful properties. In particular, it can be anticipated that such diimines, hydrazones, and amines will find use as vulcanization accelerators and stabilizers against thermal oxidation of rubber items [3–7], which makes this study topical.
EXPERIMENTAL
The organic vulcanization accelerator derived from 2-adamantanone was prepared and isolated by the procedure described in [8]. To prepare the desired bisazomethines, we performed the reaction of 2-adamantanone with 1,4-diaminobutanone (2a) and the reaction of D,L-camphor with 1,3-diaminopropane (2b) or 1,6-diaminohexane (2c). The reactions involve nucleophilic addition of amino groups of diamines to the carbonyl group with the formation of bisazomethines (Schiff bases, diimines) 3а–3с (Scheme 1).
The reactions of D,L-camphor with diamines were performed in toluene at the reactant molar ratio 1b : 2b and 1b : 2с equal to 2 : 1.25. p-Toluenesulfonic acid was used as a catalyst. The reaction completion was judged from the collection of the equimolar amount of the released water in a Dean–Stark trap. To increase the yield of bisazomethines derived from camphor (3b, 3c), an additional portion of the diamines (2b, 2c; 25% of the equimolar amount) was added to the reaction mixtures, and the reaction was continued.
The reaction mixture was washed with water, the organic layer was separated and dried over sodium sulfate, and the solvent was evaporated. The yield of the products (%) after the purification was as follows: 3а, 93; 3b, 78; and 3с, 75. The compounds obtained are resinous substances.
The compositions and structures of the compounds obtained (Table 1) were confirmed by gas chromatography–mass spectrometry. The mass spectra were recorded with an Agilent GC 5975/MSD 7820 gas chromatograph–mass spectrometer under the following conditions: HP-5MS capillary quartz column 30 m long, carrier gas helium, programmed column heating from 80 to 280°С, vaporizer temperature 250°С. Elemental analysis was performed with a Perkin Elmer Series II 2400 device.
N,N′-(Butane-1,4-diyl)bis(adamantan-2-ylimine)3а. To 7 g (0.0466 mol) of 2-adamantanone, we added 2.06 g (0.0234 mol) of 1,4-diaminobutane, 60 mL of benzene, and 0.1 g of p-toluenesulfonic acid. The reaction was performed at the boiling point of benzene (80°С). A colorless resinous substance was obtained (yield 7.68 g, 93%); its properties agree with the published data [4]. Mass spectrum, m/z (Irel, %): 338 (M, 13%), 190 (C13H20N+, 7%), 176 (C12H18N+, 100%), 162 (C11H16N+, 32%), 148 (C10H14N+, 4%). Found, %: C 78.51, H 10.79, N 6.70. C27H44N2O. Calculated, %: C 78.59, H 10.75, N 6.79.
N,N’-(Propane-1,3-diyl)bis(1,7,7-trimethylbi-cyclo[2.2.1]heptan-2-imine)3b. To 5 g (0.033 mol) of camphor, we added 1.221 g (0.0165 mol) of 1,3-diaminopropane, 30 mL of toluene, and 0.2 g of p-toluenesulfonic acid. To increase the yield of bisazomethine 3b, an additional portion (0.303 g, 0.0041 mol) of 1,3-diaminopropane was added to the reaction mixture after the water release fully ceased. The reaction was performed at the toluene boiling point (110°С). A colorless resinous substance was obtained (yield 4.4 g, 78%); its properties agree with the published data [6]. Mass spectrum, m/z (Irel, %): 342 (M, 20%), 136 (13%), 178.2 (C12H20N+, 100%). Found, %: C 80.61, H 11.12, N 8.10. C23H38N2. Calculated, %: C 80.64, H 11.18, N 8.18.
N,N'-(Hexane-1,6-diyl)bis(1,7,7-trimethylbi-cyclo[2.2.1]heptan-2-imine)3c was prepared similarly to 3b from 5 g (0.033 mol) of camphor, 1.91 g (0.0165 mol) of 1,6-diaminohexane, 40 mL of toluene, and 0.2 g of p-toluenesulfonic acid with the subsequent addition of 0.476 g (0.0041 mol) of 1,6-diaminohexane. A colorless resinous substance was obtained (yield 4.75 g, 75%); its properties agree with the published data [6]. Mass spectrum, m/z (Irel, %): 384 (M, 3%), 179 (17%), 234 (C16H28N+, 100%). Found, %: C 81.27, H 11.55, N 7.21. C26H44N2. Calculated, %: C 81.19, H 11.53, N 7.28.
The bisazomethines obtained were tested as vulcanization accelerators for elastomer compounds based on SKI-3 rubber (Table 2). To compare the properties of the vulcanizates obtained, we also used samples containing a commercial vulcanization accelerator, 2-mercaptobenzothioazole (Captax).
The rubber stocks were prepared and vulcanized in accordance with GOST (State Standard) 30263–96, Rubber Stocks for Testing. Preparation, Mixing, and Vulcanization. Equipment and Methods. Vulcanization of the rubber stock was performed in a PHG-2 212/4 vulcanization press at 155°С for 20 min.
The vulcanization characteristics of rubber stocks were determined with an MDR3000 Professional ASTM D 2084–79 rheometer. The elastic and strength properties of the vulcanized rubbers were determined according to GOST 270–75, Vulcanized Rubber. Method for Determining Tensile Elastic and Strength Characteristics. Tests for the resistance of vulcanized rubbers to thermal aging were performed according to GOST 9.024–74, Unified System for Corrosion and Aging Protection. Vulcanized Rubbers. Methods of Tests for Thermal Aging Resistance.
RESULTS AND DISCUSSION
Symmetrical bisazomethines 3b and 3с are examples of vulcanization accelerators that combine natural fragments (camphor) linked by spacer bridges.
The compounds synthesized were studied as polyfunctional ingredients in rubber stock formulations based on SKI-3 rubber.
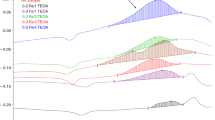
To evaluate the influence of the nature and structure of bisazomethines DADI, CD-3, and CD-6 on the vulcanization rate, we studied the vulcanization kinetics by the rheometric method (Fig. 1). Replacement of Captax by bisazomethine CD-3 increases the maximal vulcanization rate from 1.19 to 1.9 kgf min–1, i.e., by a factor of almost 2 relative to the control sample. In the presence of bisazomethine CD-6, the maximal vulcanization rate remains the same (coincidence of rate curves 1 and 4). Replacement of Captax by bisazomethine DADI decreases the maximal vulcanization rate from 1.19 to 0.73 kgf min–1.
The induction period in the presence of CD-3 decreases from 1.61 to 1.16 min, whereas replacement of Captax by DADI increases the induction period from 1.61 to 1.66 min (Table 3). In the presence of CD-6 (τS = 1.59 min), the induction period remains virtually the same as with the standard vulcanization accelerator, Captax (τS = 1.61 min).
All the bisazomethines studied, DADI, CD-3, and CD-6, showed activity as vulcanization accelerators. Equal-weight replacement of Captax by CD-3 and CD-6 increases the vulcanization rate from 0.33 to 0.35 rad (the vulcanization rate parameter increases by 6%), whereas the replacement of Captax by DADI decreases the vulcanization rate from 0.33 to 0.31 rad.
Introduction of camphor-derived bisazomethines CD-3 and CD-6 leads to an increase in the vulcanization cross-linking density: ∆M increases by 5–12%, which correlates with a decrease in the degree of swelling (Table 3).
Today, there is no common theory accounting for the mechanism of the action of the vulcanization accelerators obtained, because the nature of the intermediate compounds and the process mechanism are different for accelerators of different types [9]. Presumably, azomethine compounds first form intermediate complexes (complexes of accelerators, sulfur, and vulcanization activators) due to lone electron pairs of electrons at nitrogen atoms in the accelerator molecules. Then, at the vulcanization temperature the intermediate complex decomposes to generate active species which, in turn, attack both sulfur molecules and rubber macromolecules to form polysulfide pendant groups containing accelerator fragments [9].
The reaction of polysulfide pendant groups with unmodified rubber molecules results in cross-linking. Free vulcanization accelerators and activators play a significant role in cross-linking [9].
Our experiments have shown that, in the presence of bisazomethines CD-3 and CD-6, the vulcanizate strength increases from 16.9 for the control sample (Captax) to 20.6 MPa for the sample whose vulcanization accelerator is CD-3. The vulcanizate obtained with bisazomethine CD-6 exhibits good resistance to thermal oxidative aging (Table 3).
Camphor-derived bisazomethines CD-3 and CD-6 have better physicomechanical parameters compared to adamantanone-derived bisazomethine DADI. This can be attributed to several factors. 2-Oxoadamantyl radical, in contrast to the D,L-camphor group, has a more rigid framework; because of the presence of three cyclohexane rings, it cannot take other conformations. The conformational structure of camphor is more labile and prone to folding.
Relative ease of the conformational transformations of the camphor framework [10] favors uniform distribution in the rubber matrix. The presence of two imino groups separated by an alkylene bridge allows the formation of additional cross-links to be expected; this accounts for an increase in ∆М and a decrease in the degree of swelling relative to control samples.
As seen from the properties of the vulcanizates, the compounds synthesized act as stabilizers and antioxidants under the conditions of thermal oxidative aging.
The mechanism of the inhibition of thermal oxidative processes in polymers by azomethine compounds can be considered from the standpoint of the commonly accepted theoretical concepts of the inhibited oxidation as a free-radical process. The role of antioxidants consists in the conversion of active peroxy radicals of the substrate being oxidized to weakly active inhibitor radicals [10].
Probably, the suggested mechanism of the action of the bisazomethine compounds in thermal oxidative aging of vulcanized rubbers involves the following radical reactions (with CD-6 as an example). Free macroradicals R• generated by oxidation and degradation of the polymer chain undergo addition to the double bond of the imino group to form sterically hindered С-centered radicals (А). In the process, the formation of a weakly active N-centered radical (B) by the addition of macroradical R• to the carbon atom of the imine group cannot be ruled out (Scheme 2).
The formation of C-centered radicals (А) is the most probable. It can be associated with lower steric hindrance to the addition of macroradical R• to the nitrogen atom involved in the double bond than to the carbon atom. Probably, the formed macroradical of type А will exhibit decreased activity due to steric hindrance and low rearrangement probability, which can lead to inhibition of radical processes.
Introduction of N,N '-(propane-1,3-diyl)bis-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-imine) and N,N '-(hexane-1,6-diyl)bis(1,7,7-trimethylbicyclo[2.2.1]heptan-2-imine) favors preservation of the strength characteristics on virtually the initial level compared to the standard accelerator, Captax.
CONCLUSIONS
Framework azomethine compounds derived from 2-adamantanone, D,L-camphor, and amines of various structures, were synthesized. These are active polyfunctional ingredients of rubber stocks. The substances obtained accelerate the sulfur vulcanization of elastomer compounds based on SKI-3 rubber and act as thermal stabilizers. N,N ′-(Propane-1,3-diyl)bis(1,7,7-trimethylbicyclo[2.2.1]heptan-2-imine) exhibits the best set of vulcanization and operation properties among the compounds synthesized. In the presence of this bisazomethine, the vulcanization rate parameter increases by 6% compared to 2-mercaptobenzothiazole (Captax) widely used in rubber industry.
REFERENCES
Chicherina, G.V., Popov, Yu.V., Korchagina, T.K., and Ermakova, T.A., Russ. J. Org. Chem., 2004, vol. 40, no. 5, pp. 685–689. https://doi.org/10.1023/B:RUJO.0000043714.74375.30
Patent RU 2212399, Publ. 2003.
Ignatz-Hoover, F., Maender, O.W., and Lohr, R., Rubber World, 1998, vol. 218, no. 2, pp. 38–40.
Barbera, V., Musto, S., Infortuna, G., Cipolletti, V., Citterio, A., Sun, S., and Galimberti, M., Rubber Chem. Technol., 2018, vol. 91, no. 4, pp. 701–718. https://doi.org/10.5254/rct.18.81528
Higgins, C.L., Filip, S.V., Afsar, A., and Hayes, W., Tetrahedron, 2019, vol. 75, no. 51, pp. 130–159. https://doi.org/10.1016/j.tet.2019.130759
Siddiqui, R., Saify, Z.S., Akhter, S., Saeed, S.M.G., Haider, S., and Leghari, Q., Pakistan J. Pharm. Sci., 2018, vol. 31, no. 6, pp. 2361–2365.
Jutea, F. Masotti, V., Bessiere, J.M., Dherbomez, M., and Viano, J., Fitoterapia, 2002, vol. 73, no. 6, pp. 532–535.
Butov, G.M., Popov, O.A., Burmistrov, V.V., and Zubovich, E.A., Izv. Volgograd. Gos. Tekh. Univ., 2015, vol. 159, no. 4, pp. 14–18.
Karmanova, O.V., Popova, L.V., Poimenova, O.V., and Gusev, Yu.K., Vestn. Voronezh. Gos. Univ. Inzh. Tekhnol., 2014, no. 3, pp. 126–129. https://doi.org/10.20914/2310-1202-2014-3-126-129
Sokolova, A.S., Yarovaya, O.I., Shernyukov, A.V., Gatilov, Y.V., Salakhutdinov, N.F., Razumova, Y.V., Pokrovsky, A.G., Zarubaev, V.V., Tretiak, T.S., and Kiselev, O.I., Eur. J. Med. Chem., 2015, vol. 105, pp. 236–273. https://doi.org/10.1016/j.ejmech.2015.10.010
Funding
The study was financially supported by the Ministry of Education and Science of the Russian Federation within the framework of the base part of the government assignment for the years 2017–2019 (project 4.7491.2017/BCh), by the Russian Foundation for Basic Research and Volgograd oblast (project no. 18-43-343003), and by the Russian Federation President’s grant for young Russian candidates of sciences (MK-1802.2020.3) using the equipment purchased within the Program for Strategic Development of the Volgograd State Technical University for the years 2012–2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kochetkov, V.G., Burmistrov, V.V., D’yachenko, V.S. et al. Synthesis and Study of Framework Azomethine Compounds as Ingredients of Rubber Stocks. Russ J Appl Chem 93, 801–806 (2020). https://doi.org/10.1134/S107042722006004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042722006004X