Abstract
Using the method of self-propagating high-temperature synthesis (SHS metallurgy), cast composite materials were produced in the Cr–Ti–B system. The experiments were carried out in universal SHS reactors with an initial argon pressure of Рin = 5 MPa. Mixtures of CaCrO4, TiO2, Al, and B powders were used as batch. It was shown that by varying the mass ratio α of CaCrO4/2Al/2B and 3TiO2/4Al/6B mixtures in a batch, it is possible to significantly affect the synthesis patterns, phase composition, and microstructure of the target products. The initial mixtures are capable of burning in the range of α 0–20%. The phase separation limit occurs at α = 15%. The introduction of a highly exothermic CaO2 + Al additive into the mixture made it possible to expand the phase separation limit to α = 20%. As α increases, the amount of titanium boride in the final product increases. The resulting composite material consists of titanium-chromium boride distributed in a matrix of chromium boride. The synthesized materials were characterized by X-ray and local microstructural analysis. The structural phase states of the target products produced under various conditions were studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Titanium and chromium borides are used for the manufacture of heat-resistant, refractory and wear-resistant alloys and as a basis for cutting high-temperature materials, in cermets for nuclear technology, for the manufacture of immersion thermocouple covers, etc. AlB2 borides exhibit significant mutual solubility, which leads to the formation of extensive high temperature solid solutions [1]. The Cr–Ti–B system has a hardness higher than individual compounds and has recently been intensively studied as a promising material for nuclear energy. Ceramic based on chromium borides, especially CrB2, has unique properties: high hardness (20–22 GPa), high melting point (2200°C), good elasticity modulus (211 GPa), good oxidation resistance, high thermal conductivity, low thermal coefficient expansion, high wear resistance, and chemical inertness [2, 3]. These unique properties allow utilization of chromium boride as a material for high-temperature structural products and hard coatings on cutting tools [4]. Compared to TiB2 and CrB2, titanium-chromium diboride has a higher hardness, oxidation resistance, and wear resistance. Titanium-chromium diboride has important advantages over popular tungsten carbide materials, such as lower specific gravity, high resistance at elevated temperatures, low cost, and readily available raw materials for its production. Despite the fact that (Ti, Cr)B2 diboride has such properties as high hardness, oxidation resistance, wear resistance, thermal and electrical conductivity, its high fragility limits its use in its pure form [5].
To create these materials and their manufacturing, the most widely used methods are melting and high-temperature consolidation (sintering and hot pressing) from mixtures of metal powder and pure boron powders in vacuum at temperatures of 1800–2200°С. Titanium and chromium borides are obtained by sintering without pressure with agglomeration additives (TiSi2, CrSi2, WSi2) and without additives in the temperature range of 1450–1950°C in vacuum, as well as in argon atmosphere +5% hydrogen for 2–4 h [6]. Titanium-chromium borides are obtained by hot pressing from chromium oxide Cr2O3, titanium oxide TiO2 and boron at a temperature of 1800°C in a hydrogen medium [5].
In [7], the preparation of titanium-chromium boride is described by high-temperature mechanochemical synthesis using titanium, chromium, and magnesium polyboride MgmBn. Titanium-chromium borides are also produced by SHS metallurgy (one of the areas of self-propagating high-temperature synthesis) from mixtures based on chromium oxides (CrO3 and Cr2O3), titanium (TiO2), boron (B2O3) and aluminum [8], by SHS compaction (followed by pressing the hot end product) from mixtures based on Cr, Ti, B with the addition of TiN nanoparticles [9], by the SHS method in combination with p-HIP from mixtures of elemental powders of titanium, chromium, boron, and copper additives or niobium [10, 11]. In [12], the authors by intensification of combustion produced cast boride ceramics using a highly exothermic additive based on calcium peroxide, a mixture of elemental powders of Ti, Cr, and B. SHS metallurgy is one of the most promising methods for the synthesis of cast composite materials [13]. In this method, the temperature released during the reaction allows the products to be cast. It was decided to abandon chromium (VI) oxide CrO3 becasuse of its toxicity [14] and thermal instability. Previously, the authors conducted studies on the use of CaCrO4 as a chromium-containing agent for the production of chromium carbides and borides [15, 16]. Studies of the CaCrO4 + Al + nB → CrxBy + Al2O3 + CaO system, where the amount of boron (n) was varied to synthesize Cr2B, CrB, Cr3B4, CrB2, showed that replacing CrO3 in the initial mixture with a low-hygroscopic stable CaCrO4 allows maintaining the high energy of the initial mixture and the ability of the mixture to burn, as well as to obtain refractory chromium borides in cast form. It was found that mixtures can burn in a wide range of B content; the final product consists of a mixture of chromium borides and free aluminum [15].
The aim of the work is to study the patterns of combustion of the CaCrO4 + TiO2 + Al + B system to obtain a cast composite material based on titanium-chromium boride, and to synthesize this material by SHS metallurgy methods.
EXPERIMENTAL
Components with thermal stability and the ability to realize a high combustion temperature were selected for termite mixtures.
The calculation of the ratios of the reagents of the initial mixtures was carried out according to the following schemes of chemical conversion:
Thermodynamic calculation of the adiabatic temperature of combustion and the formation of gaseous products during combustion of the mixture based on schemes (1) and (2) depending on α, where α = [M2/(M1 + M2)] × 100%, М1 is the mass of the mixture according to the scheme (1), M2 is the mass of the mixture according to scheme (2), carried out on a personal computer using Thermo [17].
In the experiments, mixtures of powders CaCrO4 (analytical grade), TiO2 (extra pure grade), Al (ASD-1 brend) and B (SVS-M brend) with a particle size dMg ≤ 10 μm and a content of B = 85 wt % and Mg = 15 wt%. In preparing the mixtures for the experiments, the Al and B contents were adjusted taking into account the participation of Mg in the reduction of CaCrO4 and TiO2 and ensuring the calculated content of B. The initial gas pressure in the experiments was 5 MPa.
To increase the combustion temperature, a highly exothermic additive 3CaO2 + 2Al = 3CaO + Al2O3 was added to the mixture, the adiabatic combustion temperature of which at P = 5 MPa is 4290 K. As a result of combustion of the additive, oxides are formed that remain in the oxide ingot and do not enter the target product. This additive allows you to raise the synthesis temperature by more than 300°.
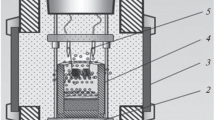
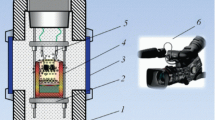
To study the combustion, the experiments were carried out in quartz glasses with a diameter of 20 mm and a height of 50 mm, the mass of the mixture was 20 g. The experiments were carried out in graphite forms with a diameter of 40 mm and a height of 100 mm for examining the technological parameters of the synthesis and final products, therewith mass of the mixture was 100 g. The studies were conducted in a 3 L reactor (Fig. 1) in an argon atmosphere with an initial gas pressure of 5 MPa. The mixture was initiated by a spiral of molybdenum wire. The combustion process was investigated using a video camera.
In the experiments, the burning rate ub, the pressure increase in the reactor ∆P, the mass loss of the mixture during combustion η1 due to the spreading, the yield of the metal phase in the ingot η2, and the completeness of the chemical reaction η3 were determined by the formulas
\( \begin{array}{*{35}{l}} {{u}_{\text{b}}}=h/t, \\ \Delta P={{P}_{\text{fin}}}-{{P}_{\text{in}}}, \\ {{\eta }_{1}}=\text{ }\left( {{M}_{\text{fin}}}-{{M}_{\text{in}}} \right)/{{M}_{\text{in}}}~\times \text{ }100, \\ {{\eta }_{2}}~={{m}_{\text{ingot}}}/{{M}_{\text{in}}}, \\ {{\eta }_{3}}={{m}_{\text{ingot}}}/{{m}_{\text{ingot}\text{.calc}}}, \\ \end{array} \)
where h is the height of the mixture layer in the quartz glass, t is the burning time, Pin and Pfin are the initial and final pressure in the reactor, Min is the mass of the initial mixture, Mfin is the mass of the final products, mingot is the mass of the metal ingot, mingot.calc is the calculated mass of the metal ingot
The burning time of the mixture was measured in two ways: by stopwatch and video; pressure was recorded by a manometer. The mass fraction of mixture 2 in the initial charge α was calculated by the formula
\( \alpha ~={{M}_{2}}/({{M}_{1}}+{{M}_{2}})\times \text{ }100, \)
where M1 is the mass of the mixture calculated according to the scheme (1), M2 is the mass of the mixture calculated according to the scheme (2).
The phase composition of the structural components was identified based on the data of X-ray phase and local microstructural analysis. X-ray phase analysis was performed on a DRON-3M diffractometer using Cu radiation with a monochromator in a secondary beam. X-Ray patterns were recorded in a step-by-step scanning mode in the range of angles 2θ = 20–80° with a shooting step of 0.02° and an exposure of 2 s. The study of the microstructure and elemental analysis of the samples was carried out on an ultra-high resolution ULTRA plus Zeiss field emission scanning electron microscope with an INCA 350 Oxford Instruments microanalysis system.
According to thermodynamic calculations (Fig. 2), with an increase in α, the combustion temperature of mixtures decreases from 2660 to 2470 K. The products of chemical transformation of the mixture are “metallic” Cr–B–Ti–Al and oxide Al2O3–B2Ca3O6 melts. With increasing α, the content of the “metallic” phase a1 of the combustion products increases, while that of the oxide a2 phase decreases (Fig. 2). The calculation showed the presence of B2Ca3O6 in the oxide phase, which indicates the participation of boron in the reduction of CaCrO4 and TiO2.
RESULTS AND DISCUSSION
Visual observations showed that after ignition a combustion front forms, which moves through the mixture. The final products in the combustion wave are obtained in the liquid-phase state and, due to different specific gravities, are divided into two layers: the lower one is “metallic” Cr–Ti–B, and the upper one is oxide Al2O3–CaO. An increase in the amount of the mixture in the initial batch according to scheme (2) led to the fact that, due to the low calculated combustion temperature, the mixtures are capable of burning in the range of α 0–20% (Fig. 3). With an increase in α, the burning rate U decreases from 11 to 7 mm s–1, and the pressure increase ΔР in the reactor also decreases from 1.35 to 0.8 MPa. The yield of the target product (η2) with increasing α passes through a maximum, and the phase separation limit occurs at α = 15%. The spreading of products (η1) practically does not change and does not exceed 3%. The completeness of the reaction (η3) also passes through a maximum at α = 5% and then decreases to 40% at α = 15%.
To expand the phase separation limit, an experiment with a highly exothermic CaO2 + Al additive was carried out. In the mixture calculated at α = 20%, the 20 wt % additive was introduced. As a result, phase separation was achieved, but the boride ingot in the form of droplets with a diameter of 1 to 5 mm was distributed in the oxide ingot and poorly separated from it.
X-ray analysis of the target products showed that at α = 10 (Fig. 4a) the product consists of a large number of phases. The main phases are titanium-chromium boride Cr0.5Ti0.5B2 and various chromium borides. Oxides of titanium and calcium, as well as phases Cr2AlB2 and Cr0.85Al0.15B2 indicate incomplete chemical transformation. The CrMoB4 phase was formed as a result of entering of initiation helix in the melt. At α = 20 (Fig. 4b), the product consists of titanium-chromium boride, chromium borides, and the Cr0.85Al0.15B2 phase. There are no oxide phases.
The results of electron microscopy are in good agreement with the results of X-ray phase analysis (Fig. 5). The tables below photographs of microstructures show the results of energy dispersive analysis of the structural components of the obtained product. On microsections of a metal product obtained from a mixture of CaCrO4 + TiO2 + Al + B with α = 10% (Fig. 5a), two main phases were revealed: the first phase (structural components 1–4) contains Cr, B, and Mo; the second phase (structural components 5–8) contains Cr, Ti, B, and Mo. Structural components 9, 10 contain Cr, Al, O, and Ti. The microsection of the product obtained at α = 20% (Fig. 5b) also shows that the product contains two phases: the first phase, the product base, contains Cr, B and a small amount of Al and Ti (structural component 1), the second phase contains Cr , Ti and B (structural components 2–4).
From the results of the thermodynamic calculation and experimental data, it follows that the change in the burning rate and the completeness of the metal phase in the ingot are in good agreement with the change in the calculated combustion temperature (Figs. 2, 3). Lowering the combustion temperature reduces the melt life time and results in diminishing the yield of the target product.
In the obtained materials, CrB2 is the main phase, in which grains of titanium-chromium boride are uniformly distributed. At α = 10% (Fig. 5a), Al2O3 oxide phase inclusions are present (structural components 9, 10). It can be seen on the microstructure of the sample at α = 20% (Fig. 5b) that the grains of titanium-chromium boride are more uniformly distributed over the sample and there are no inclusions of the oxide phase Al2O3. This is explained by a higher combustion temperature due to the use (20%) of a highly exothermic CaO2/Al additive. As a result, the life time of the melt and the completeness of the reaction of reduced titanium with boron increased, and the mutual solubility of chromium and titanium borides rose.
CONCLUSIONS
The foundations of a scientific approach for the synthesis of cast composite materials in the Cr–Ti–B system using calcium chromate as a chromium-containing agent have been developed by SHS metallurgy technique. Mixtures are capable of burning up to α = 20%, the phase separation limit occurs at α = 15%. With increasing α, the burning rate of the mixture decreases, and the yield of the metal (target) product passes through a maximum. The product of the autowave chemical transformation of the mixture is cast material, clearly divided into two layers: target and oxide. The introduction of a highly exothermic CaO2 + Al additive into the mixture made it possible to expand the phase separation limit to α = 20%. The structure and phase composition of the target products obtained at various contents of the starting components were studied. The resulting product is a composite material based on chromium diboride with uniformly distributed inclusions of titanium-chromium boride.
REFERENCES
Momozawa, A. and Telle, R., Vacuum, 2019, vol. 167, pp. 577–585. https://doi.org/10.1016/j.vacuum.2018.06.036
Jordan, L.R., Betts, A.J., Dahm, K.L., Dearnley, P.A., and Wright, G.A., Corrosion Sci., 2005, vol. 47, no. 5, pp. 1085–1096. https://doi.org/10.1016/j.corsci.2003.10.018
Samsonov, G.V., Serebryakova, T.I., and Neronov, V.A., Boridy (Borides). Moscow: Atomizdat, 1975.
Iizumi, K., Kudaka, K., Maezawa, D., Sasaki, T., J. Ceram. Soc. Japan, 1999, vol. 107, no. 1245, pp. 491–493. https://doi.org/10.2109/jcersj.107.491
Evtushok, T.M., Grigor’ev, O.N., Kostenko, A.D., Zhunkovskii, G.L., Kotenko, V.A., and Mazur, P.V., Powder Metallurgy Metal Ceram., 2005, vol. 44, no. 7–8, pp. 353–357. https://doi.org/10.1007/s11106-005-0102-6
Murthy, T.S.R.Ch., Sonber, J.K., Sairam, K., Bedse, R.D., and Chakarvartty, J.K., Mater. Today: Proceedings, 2016, vol. 3, no. 9, pp. 3104–3113. https:// doi.org/10.1016/j.matpr.2016.09.026 .
Arestov, O.V. and Ruzhitskaya, E.V., Vestnik inzhenernoi shkoly DVFU, 2012, no. 4(13), pp. 20–26.
Gorshkov, V.A. and Yukhvid, V.I., Abstract of Papers, 3-ya Mezhdunar. konf. “Materialy i pokrytiya v ekstremal’nykh usloviyakh: issledovaniya, primenenie, ekologicheskie chistye tekhnologii proizvodstva i utilizatsii izdelii” (Int. Conf. “Materials and Coatings in Extreme Conditions: Research, Application, Environmentally Friendly Technologies for the Production and Disposal of Products”), Katsiveli-Ponizovka, 2004.
Shcherbakov, V.A., Gryadunov, A.N., Sachkova, N.V., and Samokhin, A.V., Lett. Mater., 2015, vol. 5, no. 1, pp. 20–23. https://doi.org/10.22226/2410-3535-2015-1-20-23
Ziemnicka-Sylwester, M., Ceram. Mater. Energy Applications IV, 2014, vol. 35, no. 7, pp. 127–138. https://doi.org/10.1002/9781119040323.ch12
Xu, Q., Zhang, X.H., Han, J.C., and He, X.D., Key Eng. Mater., 2007, vol. 280–283, pp. 1441–1444. https://doi.org/10.4028/www.scientific.net/KEM.280-283.1441
Andreev, D.E, Sanin, V.N., Yukhvid, V.I., and Kovalev, D.Yu., Combustion, Explosion, and Shock Waves, 2011, vol. 47, no. 6, pp. 671–676. https://doi.org/10.1134/S0010508211060074
Levashov, E.A., Rogachev, A.S., Kurbatkina, V.V., Maksimov, Yu.M., and Yukhvid, V.I., Perspektivnye materialy i tekhnologii samorasprostranyayushchegosya vysokotemperaturnogo sinteza (Promising Materials and Technologies for Self-Propagating High-Temperature Synthesis), Moscow: Izd. Dom MISiS, 2011.
Salnikow, K. and Zhitkovich, A., Chem. Res. Toxicol., 2008, vol. 21, pp. 28–44. https://doi.org/10.1021/tx700198a
Miloserdov, P.A., Yukhvid, V.I., Gorshkov, V.A., Ignat’eva, T.I., Semenova, V.N., and Shchukin, A.S., Combustion, Explosion, and Shock Waves, 2017, vol. 53, no. 6, pp. 665–668. https://doi.org/10.1134/S0010508217060065
Miloserdov, P.A., Yukhvid, V.I., Gorshkov, V.A., Kovalev, I.D., and Miloserdova, O.M., Int. J. Self-Propagating High-Temperature Synthesis, 2018, vol. 27, no. 2, pp. 123–126. https://doi.org/10.3103/S1061386218020139
Shiryaev, A.A., Int. J. Self-Propagating High-Temperature Synthesis, 1995, vol. 4, no. 4, pp. 351–362.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ACKNOWLEDGMENTS
To perform the research, the equipment of the ISMAN Distributed Center for Collective Use was involved.
FUNDING
This work was financially supported by the grant of the Russian Foundation for Basic Research no. 18-08-00804.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Miloserdov, P.A., Gorshkov, V.A., Sachkova, N.V. et al. Synthesis of Composite Materials in the System Cr–Ti–B by the Self-Propagating High-Temperature Synthesis from Mixtures CaCrO4/TiO2/Al/B. Russ J Appl Chem 93, 362–368 (2020). https://doi.org/10.1134/S1070427220030076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220030076