Abstract
The extraction of lanthanides(III), uranium(VI), and thorium(IV) ions from nitric acid solutions with diphenyl(dibutylcarbamoylmethyl)phosphine oxide solutions significantly increases in the presence of ionic liquids, 1-butyl-3-methylimidazolium dialkylsulfosuccinates, in the organic phase. The dependence of the ionic liquid distribution between the organic and aqueous phases on the concentrations of the ionic liquid and nitric acid was studied. The stoichiometry of the extracted complexes of lanthanides(III), uranium(VI), and thorium(IV) was determined by the equilibrium shift method. The influence of the length of alkyl radicals in the ionic liquid anionic part and the HNO3 concentration in the aqueous phase on the efficiency of metal ions extraction into the organic phase containing the ionic liquid was considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Extraction methods are widely used in the processing of spent nuclear fuel to extract, concentrate, and separate actinides and lanthanides [1]. Neutral bidentate organophosphorus compounds, in particular, diaryl(dialkylcarbamoylmethyl)phosphine oxides, have a high extraction ability with respect to actinide and lanthanide (Ln) ions in nitric acid media [2–6]. Recently, the possibility of using ionic liquids in extraction practice as a water-immiscible phase in the extraction of metal ions has been considered [7–14]. Actinide and Ln(III) ions are extracted with solutions of carbamoylmethylphosphine oxides in such ionic liquids, as 1-alkyl-3-methylimidazolium hexafluorophosphates and bis[(trifluoromethyl)sulfonyl]imides, much more efficiently than with solutions of carbamoylmethylphosphine oxides in traditional solvents [14]. A rather low ionic liquid concentration in an organic solvent containing carbamoylmethylphosphine oxide is sufficient to extract efficiently lanthanides and actinides from solutions in HNO3 or HCl [15].
As a rule, ionic liquids based on 1-alkyl-3-methylimidazolium cations ([Cnmim]+) were used for the extraction of metal ions. An increase in the length of the alkyl radical in the cation leads to a decrease in the efficiency of actinides and Ln(III) extraction in the carbamoylmethylphosphine oxide–ionic liquid system [14]. The use of bis[(trifluoromethyl)sulfonyl]imide (Tf2N‒) as an ionic liquid anionic component is caused by its high hydrophobicity and hydrolytic and radiolytic stability in nitric acid media compared to the hexafluorophosphate anion [7]. The trend towards the use in extraction practice of ionic liquids with anions free from fluorine, in particular, dialkyl sulfosuccinates, is associated with their wider availability and lower toxicity [16].
We have studied the influence of the nature of the anionic part of ionic liquids on the efficiency of lanthanides(III), uranium(VI), and thorium(IV) extraction from nitric acid solutions with solutions of diphenyl(dibutylcarbamoylmethyl)phosphine oxide (L) in an organic solvent. For this purpose, we have considered the interfacial distribution of Ln(III), U(VI), and Th(IV) between HNO3 solutions and an organic phase containing compound L and 1-butyl- 3-methylimidazolium di-2-ethylhexyl- (1) or dihexyl- (2) sulfosuccinates (Scheme 1).
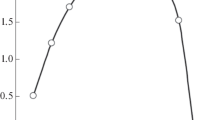
To form an estimate of the hydrophobicity of ionic liquids 1 and 2, we considered the distribution of the anions of these liquids between dichloroethane and the aqueous phase. An increase in the concentration of the ionic liquid in the organic phase is accompanied by an increase in the distribution coefficients of anions (DA‒) (Fig. 1).
The equilibrium between the components of the organic and aqueous phases can be described by Eq. (1).
Here A– is the anion of the ionic liquid; the symbols (o) and (aq) refer to the components of the system in the organic and aqueous phases, respectively.
The concentration constant of the ionic liquid distribution between the organic and aqueous phases has form (2).
The concentration constants of the distribution of ionic liquids 1 and 2 between dichloroethane and water calculated according to equation (2) with the data of Fig. 1 are (3.02±0.17)×105 (R2 0.998) and (1.32±0.08)× 104 (R2 0.997), respectively. Thus, an increase in the length of alkyl substituents in the anionic part of an ionic liquid leads to an increase in its hydrophobicity. The hydrophobicity of ionic liquids 1 and 2 is significantly higher than that of ionic liquids with the Tf2N‒ anion (the C4mimTf2N distribution constant between dichloroethane and water is 7940 [17]). Therefore, the loss of ionic liquids based on dialkyl sulfosuccinate anions during the extraction of metal ions is much less. An increase in the HNO3concentration in the aqueous phase leads to a decrease in the DA‒ distribution coefficient (Fig. 2), which was noted earlier in the Tf2N‒ anion distribution between the organic and aqueous phases [17]. This is due to the C4mimNO3 formation in nitric acid media.
In contrast to imidazolium ionic liquids with Tf2N‒ anions, ionic liquids 1 and 2 with dialkylsulfosuccinate anions are able to extract metal ions from neutral and slightly acidic solutions [18]. The ability of ionic liquid 1 to extract metal ions sharply decreases with increasing acidity of the aqueous phase (Fig. 3), and at [HNO3] > 2 M solutions of compound 1 in dichloroethane practically do not extract Ln(III), U(VI), and Th(IV) from nitric acid solutions (distribution coefficients D do not exceed 10‒2). However, during extraction with mixtures of compounds L and 1 in dichloroethane, a significant increase in the degree of extraction of these elements into the organic phase occurs (Fig. 3). The observed synergistic effect can result from the incorporation of hydrophobic dialkylsulfosuccinate anions in the extractable complexes, which leads to an increase in their hydrophobicity compared to solvated Ln(III), U(VI), and Th(IV) nitrates extracted by solutions of compound L from nitric acid solutions.
Dependence of the distribution coefficients of (1–3) U(VI) and (4–6) Eu(III) on the HNO3 concentration in the equilibrium aqueous phase during extraction with solutions of compounds (2, 5) L and (3, 6) 1 in dichloroethane and (1, 4) solutions of L in dichloroethane containing 0.1 M ionic liquid 1. Concentration of L, M: (4, 5) 0.02; (1, 2) 0.005.
The presence of ionic liquid 1 in the organic phase significantly changes the nature of the dependence of the distribution coefficients of metal ions on the HNO3 concentration in the aqueous phase (Fig. 3). During extraction with solutions of L in dichloroethane, the DU values increase symbately with the HNO3 concentration, which corresponds to the extraction of metal ions by the solvate mechanism. A slight decrease in the DEu value at [HNO3] > 3 M is associated with a decrease in the concentration of the free extractant in the organic phase due to the joint extraction of nitric acid [5]. During the extraction of metal ions with L solutions in the presence of ionic liquid 1, D decreases as the HNO3 concentration increases (Fig. 3). The same character of the D dependence of on [HNO3] was observed in the extraction of Ln(III), U(VI), and Th(IV) with mixtures of L and C4mimTf2N in dichloroethane [15]. This is due to the change in the mechanism of metal ions extraction by neutral donor-active extractants from solvate to cation-exchange in systems with ionic liquids (3).
Here s is the solvation number.
The value of the synergistic effect S = D/(DL + DIL) (DL, D1, and D are the distribution coefficients for extraction with individual ligand, ionic liquid 1, and their mixtures, respectively) decreases with increasing HNO3 concentration in the aqueous phase. During the extraction of Eu(III) with mixtures of L and liquid 1, an increase in the HNO3 concentration from 0.5 to 5 M is accompanied by a decrease in the S value from 1380 to 16.6.
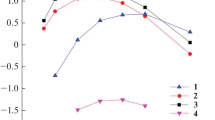
The nature of the anion of the ionic liquid has a significant effect on the efficiency of the metal ions extraction from nitric acid solutions with mixtures of L and the ionic liquid. During the extraction of Ln(III) with solution of L in the presence of more hydrophobic ionic liquid 1, a more noticeable increase in the distribution coefficients of Ln(III) is observed than in the system with liquid 2 (Fig. 4). Under comparable conditions, the S values of the Eu(III) extraction are 40 and 4.5 in systems with liquids 1 and 2, respectively. A tendency for a decrease in the efficiency of Ln(III) extraction with an increase in the atomic lanthanide number (Z) is observed during the extraction with solutions of L, as well as with mixtures of L and ionic liquid 1 from nitric acid solutions (Fig. 4). This is caused by the increase in the hydration energy of Ln3+ ions as the ionic radii decreases with increasing Z [4].
An increase in the compound 1 concentration in the organic phase is accompanied by an increase in DLn (Fig. 5). At [1] > 0.02 M, an increase in the concentration of the ionic liquid less noticeably affects the DLn increase, whereas at [1] > 0.1 M, some DLn decrease is observed. Similar dependences were observed earlier in the extraction of Ln(III) with solutions of carbamoylmethylphosphine oxides in the presence of C4mimTf2N [18]. One of the reasons for this phenomenon may be the solvation of the ionic liquid cation by a molecule of the neutral extractant in the organic phase, which leads to a decrease in the concentration of the free extractant in the organic phase [19].
The stoichiometric metal–L ratio in the Ln(III), U(VI), and Th(IV) complexes extracted in the presence of ionic liquid 1 was determined by the equilibrium shift method. At a constant concentration of HNO3 in the aqueous phase and ionic liquid 1 in the organic phase, the angular slope of the log DLn – log [L] dependences is close to 2 (Fig. 6), which points to the extraction of Ln(III) ions from nitric acid solutions in the form of disolvates. Under similar conditions, Th(IV) is also extracted in the form of disolvates (Fig. 6). In the case of U(VI) extraction, the angular slope of the log DU–log [L] dependences is 1.41±0.12 (R2 0.998), which indicates that U(VI) is extracted as a mixture of mono- and disolvates. Previously, it was shown that in the L‒C4mimTf2N system, the stoichiometric ratios of U(VI) : L and Th(IV) : L are close to 1 : 2, and Ln(III) is extracted as complexes of the composition 1 : 2 and 1 : 3 [15]. Apparently, the increase in the solvation numbers in the Ln(III) and U(VI) complexes extracted in the L‒C4mimTf2N system is associated with the weak coordinating ability of the Tf2N‒ ions [20], which are apparently located in the outer coordination sphere of the extracted complex. On the contrary, dialkylsulfosuccinate anions can participate in the coordination of metal ions [18].
The presented data showed that the efficiency of Ln(III), U(VI), and Th(IV) extraction from nitric acid solutions with diphenyl(dibutylcarbamoylmethyl)phosphine oxide solutions significantly increases in the presence of ionic liquids, 1-butyl-3-methylimidazolium dialkylsulfosuccinates, in the organic phase. An increase in the hydrophobicity of anions in ionic liquids is accompanied by an increase in the efficiency of metal ions extraction from nitric acid solutions. The increase in the hydrophobicity of 1-butyl-3-methylimidazolium dialkylsulfosuccinates compared to the ionic liquid based on the bis[(trifluoromethyl)sulfonyl]imide anion causes a significantly lower transition of these liquids into the aqueous phase during extraction.
EXPERIMENTAL
The synthesis of diphenyl(dibutylcarbamoylmethyl)phosphine oxide was described earlier [21]. 1-Butyl3-methylimidazolium dihexyl- and di-2-ethylhexylsulfosuccinates were obtained by reacting 1-butyl-3-methylimidazolium bromide with sodium dihexyl- or di(2-ethylhexyl)sulfosuccinates [22]. Chemically-pure grade 1,2-dichloroethane grade was used as an organic solvent.
The initial aqueous solutions of Ln(III), U(VI), and Th(IV) were prepared by dissolving corresponding nitrates in water followed by the HNO3 addition. The initial concentration of metal ions was 10–5 M. The phases were contacted for 1 h at room temperature on a rotary apparatus, 60 rpm. It has been previously found that this time is sufficient to reach constancy of the distribution coefficients of the elements in extraction systems.
The concentrations of Ln(III), U(VI), and Th(IV) in initial and equilibrium aqueous solutions were determined by inductively coupled plasma mass spectrometry on an XSeries II mass spectrometer (Thermo Scientific, USA). The amount of elements in the organic phase was determined from the difference between the concentrations in the aqueous phases before and after extraction. The distribution coefficients of the elements were calculated from the ratio of their concentrations in the equilibrium organic and aqueous phases. The error in determining the distribution coefficients did not exceed 5%. The concentration of HNO3 in the equilibrium aqueous phase was measured by potentiometric titration with a NaOH solution. The concentration of dialkylsulfosuccinate ions in equilibrium aqueous phases was determined by the atomic emission method with sample ionization in inductively coupled plasma on an ICAP-61 spectrometer (Thermo Jarrel Ash, USA).
REFERENCES
Myasoedov, B.F. and Kalmykov, S.N., Mendeleev Commun., 2015, vol. 25, no. 5, p. 319. https://doi.org/10.10016/j.mencom.2015.09.001
Leoncini, A., Huskens, J., and Verboom, W., Chem. Soc. Rev., 2017, vol. 46, p. 7229. https://doi.org/10.1039/C7CS00574A
Alyapyshev, M.Yu., Babain, V.A., and Ustynyuk, Yu.A., Russ. Chem. Rev., 2016, vol. 85, no. 9, p. 943. https://doi.org/10.1070/RCR4588
Horwitz, E.P., Martin, K.A., Diamond, H., and Kaplan, L., Solv. Extr. Ion Exch., 1986, vol. 4, no. 3, p. 449. https://doi.org/10.1080/07366298608917877
Chmutova, M.K., Litvina, M.N., Pribylova, G.A., Ivanova, L.A., Smirnov, I.V., Shadrin, A.Yu., and Myasoedov, B.F., Radiochem., 1999, vol. 41, no. 4, p. 331.
Safiulina, A.M., Lizunov, A.V., Borisova, N.E., Baulina, T.V., Goryunov, E.I., Goryunova, I.B., and Brel, V.K., Russ. J. Inorg. Chem., 2021, vol. 66, no. 5, p. 731, p. 414. https://doi.org/10.1134/S0036023619030057
Kolarik, Z., Solv. Extr. Ion Exch., 2013, vol. 31, p. 24. https://doi.org/10.1080/07366299.2012.700589
Shkrob, I.A., Marin, T.W., and Jensen, M.P., Ind. Eng. Chem. Res., 2014, vol. 53, p. 3641. https://doi.org/10.1021/ie4036719
Iqbal, M., Waheed, K., Rahat, S.B., Mehmood, T., and Lee, M.S., J. Radioanal. Nucl. Chem., 2020, vol. 325, p. 1. https://doi.org/10.1007/s10967-020-07199-1
Okamura, H. and Hirayama, N., Anal. Sci., 2021, vol. 37, p. 119. https://doi.org/10.2116/analsci.20SAR11
Belova, V.V., Radiochem., 2021, vol. 63, p. 1. https://doi.org/10.1134/S106636222101001X
Quijada-Maldonado, E., Olea, F., Supulveda, R., Castillo, J., Cabezos, R., Merlet, G., and Romero, J., Sep. Pur. Technol., 2020, vol. 251. 117289. https://doi.org/10.1016/j.seppur.2020.117289
Atanassova, M., J. Mol. Liq., 2021, vol. 343, article 117530. https://doi.org/10.1016/j.molliq.2021.117530
Sun, T., Zhang, Y., Wu, Q., Chen, J., Xia, L., and Xu, C., Solv. Extr. Ion Exch., 2017, vol. 35, p. 408. https://doi.org/10.1080/07366299.2017.1379142
Turanov, A.N., Karandashev, V.K., and Yarkevich, A.N., Radiochem., 2013, vol. 55, no. 4, p. 382. https://doi.org/10.1134/S1066362213040073
Dib, N., Dario Falcone, R., Acuna, A., and Garcia-Rio, L., J. Mol. Liq., 2020, vol. 304. 112762. https://doi.org/10.1016/j.molliq.2020.112762
Turanov, A.N., Karandashev, V.K., and Baulin, V.E., Solv. Extr. Ion Exch., 2008, vol. 26, p. 77. https://doi.org/10.1080/07366290801904871
Vendilo, A.G., Djigailo,, D.I., Smirnova, S.V., Torocheshnikova, I.I., Popov, K.I., Krasovsky, V.G., and Pletnev, I.V., Molecules, 2009, vol. 14, p. 5001. https://doi.org/10.3390/molecules144125001
Turanov, A.N., Karandashev, V.K., and Baulin, V.E., Solv. Extr. Ion Exch., 2012, vol. 30, p. 244. https://doi.org/10.1080/07366299.2011.639248
Binnemans, K., Chem. Rev., 2007, vol. 107, p. 2593. https://doi.org/10.1021/cr050979c
Turanov, A.N., Karandashev, V.K., Kharitonov, A.N., Lezhnev, A.N., Safronova, Z.V., Yarkevich, A.N., and Tsvetkov, E.N., Russ. J. Gen. Chem., 1999, vol. 69, no. 7, p. 1068.
Brown, P., Butts, C.P., Eastoe, J., Fermin, D., Grillo, I., Lee, H.-C., Parker, D., Plana, D., and Richardson, R.M., Langmuir, 2012, vol. 28, p. 2502. https://doi.org/10.1021/la204557t
Funding
The work was carried out within the framework of the State Assignment for 2021 of the Yu.A. Osipyan Institute of Solid State Physics, RAS, Institute of Problems of Microelectronics Technology and Highly Pure Materials, RAS, and Institute of Physiologically Active Substances, RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Turanov, A.N., Karandashev, V.K., Burmii, Z.P. et al. Effect of Dialkylsulfosuccinate-Based Ionic Liquids on the Extraction of Lanthanides(III), Uranium(VI), and Thorium(IV) with Diphenyl(dibutylcarbamoylmethyl)phosphine Oxide from Nitric Acid Solutions. Russ J Gen Chem 92, 418–423 (2022). https://doi.org/10.1134/S1070363222030082
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222030082