Abstract
The surface properties of membranes with a dynamic layer of cellulose acetate have been investigated, and the parameters for the purification of oily wastewater have been established. A polytetrafluoroethylene microfiltration membrane was used a substrate, on the surface of which a 42‒128-nm dynamic layer of cellulose acetate particles was formed. Changes in the surface properties of the membranes were studied by scanning electron microscopy, and the wettability of the membrane surface was determined by the recumbent drop method. The retention capacity of the membranes was determined using the oil products contained in a model waste water and in 0.1% oil emulsion. The retention capacity of the membrane with respect to oil products from wastewater was estimated at up to 98%, with the specific productivities with respect to wastewater of 1485 dm3 m–2 h–1 and with respect to 0.1% oil emulsion of 219 dm3 m–2 h–1. Such purification parameters are not inferior to those of commercial ultrafiltration membranes. The concentration of oil products after cleaning does not exceed the maximum allowable levels for wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Pollution of the natural environment with oil and oil products poses a serious threat to aquatic ecosystems, primarily aquatic organisms. Increasing production and, as a consequence, increasing scale of transportation, processing, and consumption of oil and its derivatives lead to regional and global environmental pollution.
Wastewater containing emulsified and dissolved oil products is quite difficult to treat because of the high stability of emulsions, and, therewith, the methods of sedimentation, flotation, and coagulation and the use of oil traps do not effectively remove oil products from water. Therefore, a demand for more efficient methods of wastewater treatment arises. One of such methods is the membrane method. Membranes are widely used for the separation of water mixtures of various origins and composition in chemical, petrochemical, food, electronic, gas, pharmaceutical, microbiological, and nuclear industries, agriculture, medicine, and water treatment for various purposes, etc.
The main properties of membrane processes for the separation of water mixtures, which distinguish them from other purification methods, are the simplicity of apparatus design, reliability, small operating costs, minimized mass characteristics, environmental safety, and high efficiency. All of these properties make it possible to purify water to such an extent that it can be reused in the technological process. The main purpose of commercial membranes is water treatment for various industries: municipal, food, medical, energy, and mechanical engineering.
Commercial membranes for wastewater treatment are either absent or quite few in number. The known commercial membrane elements for wastewater treatment have a number of disadvantages: high cost of treatment and dependence of membrane performance on the level of wastewater pollution [1]. When treating highly contaminated wastewater one can face the phenomenon of concentration polarization, which will entail a decrease in performance and potential irreversible changes in the surface properties of the membranes, and, as a result, will require regeneration or replacement of membrane elements.
Thus, to develop new membranes for the purification of natural and waste waters from oil products, which have a high retention capacity and permeability, resistance to surface pollution, and a low production cost is an urgent task.
Today, composite membranes for ultrafiltration and nanofiltration are widely used [2–4]. Composite membranes have advantages in terms of cost, production method, and the possibility of imparting some desired properties to the membranes by applying on their surface an ultrathin layer of a functional material. As a result, the specific performance increases, the wettability changes, and the chemical and mechanical strength increases [5, 6]. Jimoh et al. [7] prepared a composite membrane by modifying a polyethersulfone hollow fiber membrane via precipitation of calcium carbonate.
The aragonite-precipitated calcium carbonate was obtained by a reaction-assisted synthesis using dolomite and an aloe vera extract. The modification increased the efficiency of the membrane in removing oil products from wastewater to 99%. For efficient separation of wastewater containing emulsified oil products, Gabor et al. [8] obtained a cotton composite membrane with one side hydrophilic and the other side hydrophobic. The efficiency of this membrane in the separation of water–oil mixtures was 1.5–2 times higher compared to a one-sided hydrophilic membrane.
In our recent study [9], commercial PTFE and polyethersulfone ultrafiltration membranes with a cut-off particle size of 100 KDa were modified with titanium oxide for use to purify wastewater containing oil products. A was found for Membranes modified with nanomaterials showed high filtration rates. The oil removal efficiency was 83–91% for commercial and more than 98% for modified membranes with a specific performance of 301–362 dm3 m–2 h–1. However, the use of an ultrafiltration membrane as a substrate for modification increases the cost of such membranes. Therefore, composite membranes are less expensive to produce on the basis of microfiltration membranes or porous filtration materials.
The proposed composite membranes open up new perspectives in the development of an ideal membrane for water purification from oil products and thus to resolve the trade-off between a high specific performance and a high retention capacity and the resistance of membranes to fouling. Therefore, the main task in the development and production of ultrafiltration membranes for emulsion separation is to approach the problem of the concentration polarization and contamination of the membrane surface with oil.
The aim of the present work was to develop a method for producing an inexpensive dynamic ultrafiltration membrane for the purification of oily wastewater and for the separation of oil emulsions.
EXPERIMENTAL
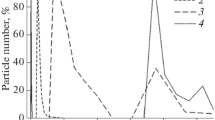
As a substrate for preparing the target membrane we chose hydrophilic polytetrafluoroethylene (PTFE) microfiltration membrane with an average pore size of 0.45 μm, brand MFFK-3G, manufacturer Vladipor (Vladimir). Such substrate material was chosen for the strength, chemical resistance, and inertness of PTFE. The dynamic layer of the membrane was formed from CA particles of the corresponding phase of the suspension. To prepare a CA suspension, we first prepared a solution of CA in acetone. The resulting solution was then sequentially diluted by half distilled water to obtain 0.5 and 5% CA suspensions in aqueous acetone. The graphs of the particle size distribution of the CA suspension in aqueous acetone, measured by dynamic light scattering on a Brookhaven Instruments NanoBrook Omni analyzer, are shown in Fig. 1.
It was found that the CA particle size in the suspension correlates with the concentration of CA in the initial solution. At a higher concentration of CA, the particle size spans the range 317–437 nm. The pore sizes of the dynamic membrane depend on the particle size in the dynamic layer; therefore, we took a 0.5% CA suspension with the particle size 42–128 nm to prepare the membrane. The dynamic membrane was prepared by forming a semipermeable layer on the surface of the porous substrate from the suspended CA microparticles present in the filtered suspension in the dynamic equilibrium with the solution.
The percentage of CA in the membrane was determined gravimetrically from the weight of the membrane before and after modification using an Adventurer RV 214 analytical balance.
The contact angles of the surface of the initial and dynamic membranes with distilled water were determined by the recumbent drop method using a Kruss DSA 20E analyzer.
The total porosity of the membranes was determined by weighing before and after impregnation with distilled water.
The micrographs of the initial substrate and dynamic membrane were examined using a Zeiss LEO-1430 VP scanning electron microscope (SEM).
The process of the purification of oily wastewater and separation of oil emulsions with dynamic and commercial ultrafiltration membranes was implemented on a laboratory membrane separation unit comprising a container with the initial liquid, a liquid pump, a pressure gauge, a membrane element, a pressure regulator, and a container for collecting the filtrate. The initial emulsion is separated by a membrane into a filtrate and a concentrate, and the concentrate is returned back to the container with the initial liquid. The process of membrane separation was carried out the following conditions: 1) determination of the specific performance of the membranes with respect to distilled water: working pressure 0.35 MPa, liquid temperature –24.0°С; separation of oil emulsion and oily wastewater: working pressure 0.30 MPa; liquid temperature –24.0°С; degree of permeate recovery 70%.
The specific performance of the membranes in the removal of oil products from wastewater was assessed using a model wastewater containing dissolved oil products and a model 0.1% oil emulsion. The model oily wastewater with a concentration of petroleum products of 25.6 mg/dm3 was prepared from a standard sample of petroleum products in a water-soluble matrix (State Standard Sample 7117-94). A 0.1% oil emulsion was obtained by dispersing carbonic oil in distilled water in the presence of sodium dodecyl sulfate. The emulsion was prepared from the carbonaceous oil extracted by the Leninogorskneft Oil and Gas Production Division. The retention capacity of the membrane was assessed in terms of “oil products” parameter. The latter parameter was calculated as the ratio of the concentrations of oil products before and after separation, which was measured on a KN-3 concentration meter.
RESULTS AND DISCUSSION
By forming a dynamic layer from a 0.5% suspension of CA in acetone on a substrate of a PTFE microfiltration membrane we obtained the dynamic membranes PTFEg–CAd with CA concentrations ranging from 3 to 9 wt%, depending on the number of dynamic layers (Table 1).
As seen from Table 1, after the first dynamic CA layer had been deposited on the substrate, the CA concentration was 3%; each successive layer increased the CA concentration by 3%.
The obtained dynamic membranes were then tested, and their properties were compared with those of commercial ultrafiltration membranes. The results are presented in Table 2.
The deposition of dynamic layers of CA particles on the surface of a hydrophilic PTFE membrane increases the hydrophilic properties of the membrane, as evidenced by the increased porosity (up to 14%) and moisture capacity (up to 20%). The decrease in the contact angle of the initial PTFE membrane of about 16° after the deposition of three CA layers on its surface was revealed. This finding is explained by the hydrophilic properties of CA particles. For comparison we chose an ultrafiltration membrane of the UPM-100 brand, manufacturer Vladipor (Vladimir). It was found that the commercial membrane UPM-100 was inferior in porosity and moisture capacity than the dynamic membranes.
The electron micrographs of the initial PTFE substrate and the PTFEg-Cad dynamic membrane at a magnification of 4000x are shown in Fig. 2.
The initial hydrophilic PTFE substrate has a smooth surface with oval pores (Fig. 2a). The deposition of 42–128- nm CA particles from the suspension form on the surface and in the pores of the substrate a dynamic layer containing a great number of, the sizes of which are an order of magnitude smaller than that of the initial substrate (Fig. 2b). The micrograph of the obtained membrane shows that the pores are evenly distributed over the entire surface, unlike the initial membrane.
Further on we investigated the specific performance of the membranes with respect to distilled water, oily waste water, and a model 0.1% oil emulsion. The resulting data are listed in Table 3.
The specific performance of the PTFE microfiltration membrane with respect to distilled water is 19998 dm3 m–2 h–1 at an operating pressure of 0.35 MPa. After the formation of dynamic layers from CA particles, the specific performance of the membrane decreases, and each subsequent layer decreases specific productivity with respect to distilled water 1.8–3.7 times. The performance of the commercial membrane is 1843 dm3 m–2 h–1, which is between the performance of the dynamic membranes PTFEg-CAd2 and PTFEg-CAd3. The performance of the membranes with respect to the model oil-containing water with the oil concentration of 25.6 mg/dm3 decreases insignificantly, no more than 4% for the dynamic membranes and up to 6% for the commercial membrane (cf. with the respective values for distilled water). By contrast, when it comes to the separation of the oil emulsion, the specific performance of the microfiltration membranes PTFE (MFFK-3G) and PTFEg-Cad decreases 19–22 times and that of the ultrafiltration membranes decreases 6.5–7.5 times.
The efficiency of the purification of wastewater was assessed in terms of the “oil products” parameter; simultaneously, the retention capacity with respect to oil products was established (Table 4).
The concentration of oil products in the filtrates of oil-containing wastewater filtered through membranes decreases; the retention capacity of the initial PTFEg membrane is 64.5%. Modification increases of the efficiency of the membranes in the removal of oil products from water. For example, the retention capacity of a three-layer dynamic membrane has increased by 32.5% to reach 97%, which is much better than with the UPM-100 commercial ultrafiltration membrane. After purification with all the modified membranes, the concentration of oil products in the filtrates is not higher than the maximum allowable concentrations for wastewater, set by the Decree of the Government of the Russian Federation no. 1134 of 03.11.2016.
It is known that oily waters form emulsions that are difficult to purify due to stability, high concentration of oil products, and high degree of dispersion of oil particles [10]. In this regard, we studied the retention capacity of the obtained membranes with respect of oil products from the emulsion. To select membranes with the required pore size, we determined the particle size of the disperse phase of the oil emulsion. The particle size distribution of the disperse phase of the 0.1% oil emulsion is presented in Fig. 3.
The model 0.1% oil emulsion is a polydisperse system, the particle sizes in the disperse phase span the range 228‒244 nm, the ζ potential of the emulsion is 23.3 mV, and the concentration of oil products in the emulsion is 802 mg/dm3. For efficient removal of oil particles from the emulsion, a membrane with an average particle size of ≤0.22 µm is required. We tested in our work a microfiltration membrane with an average pore size of 0.45 µm, commercial membrane UMP-100 with a molecular weight cut-off of 100 kD, and dynamic membranes.
As seen from Table 5, the retention capacity of the dynamic membranes increases with increasing number of CA layers. Thus, the initial microfiltration membrane is capable of retaining up to 48.0% of oil products from a 0.1% oil emulsion. The deposition of one CA layer on the surface of initial membrane increases its retention capacity to 98.8%, while that of the commercial membrane with one CA layer is 97.3%. The concentration of oil products is consistent with the MAC only in the filtrate of the PTFEg–CAd3 dynamic membrane.
After separation of the emulsion, optical photographs of 1% oil emulsion and its filtrates were obtained at a magnification of 1000 times (Fig. 4).
From Fig. 4 it follows that 0.1% oil emulsion consists of spherical oil particles. After separation of the emulsion with a PTFEg microfiltration membrane (MFFK-3G), the number of oil particles in the field of view decreased (Fig. 4b), and after separation with a dynamic PTFEg-CAd membrane, there are no oil particles in the field of view at all.
Thus, based on the studies carried out, it has been shown that dynamic membranes with a surface layer of CA particles can effectively remove oil products from wastewater, including from stable emulsions.
CONCLUSIONS
Dynamic membranes with a surface layer of CA particles have been proposed for wastewater treatment and oil emulsion separation. The deposition of CA surface layers on a membrane increases the hydrophilic properties of the membrane surface. According to the micrograph of the obtained membranes, the surface layer is covered with a dynamic layer with a great number of pores, the sizes of which are an order of magnitude smaller than that in the initial membrane, and the pores are evenly distributed over the entire surface, unlike the original membrane substrate. Dynamic membranes can only be applied in oily wastewater treatment processes, as they demonstrate a high retention capacity with respect to both dissolved and emulsified oil products. A dynamic membrane with three CA surface layers showed high efficiency in the treatment of oily wastewater and in the separation of oil emulsion, with a retention capacity with respect to oil products of up to 98% and a specific performance with respect to wastewater of 1485 dm3 m–2 h–1 and with respect to a 0.1% oil emulsion of 219 dm3 m–2 h–1 (at 0.35 MPa), which is better than the respective parameters of the commercial ultrafiltration membrane UPM-100.
REFERENCES
Fazullin, D.D., Mavrin, G.V., Shaikhiev, I.G., and Ziganshin, A.F., Vestn. Tekhnol. Univ., 2017, vol. 20, no. 4, p. 127.
Fazullin, D.D., Mavrin, G.V., and Salakhova, A.N., Membr. Membr. Technol., 2020, vol. 2, no. 2, p. 115. https://doi.org/10.1134/S2517751620020067
Fazullin, D.D., Mavrin, G.V., and Shaikhiev, I.G., Petrol. Chem., 2018, vol. 58, no. 2, p. 145. https://doi.org/10.1134/S0965544117130047
Fazullin, D.D., Mavrin, G.V., Fazullina, L.I., and Nasyrov, I.A., Helix, 2019, vol. 9, no. 5, p. 5563. https://doi.org/10.29042/2019-5563-5567
Ding, Y., Wu, J., and Wang, J., J. Membr. Sci., 2020, vol. 614, article no. 118491. https://doi.org/10.1016/j.memsci.2020.118491
Maryam, A., Nabian, N., and Delavar, M., Sep. Purif. Technol., 2020, vol. 251, article no. 117332. https://doi.org/10.1016/j.seppur.2020.117332
Jimoh, O. A., Okoye, P. U., Otitoju, T.A., and Ariffin, K.A., J. Clean. Prod., 2018, vol. 195. p. 79. https://doi.org/10.1016/J.JCLEPRO.2018.05.192
Hu, L., Liu, Y., and Wang, Z., CAS Appl. Nano Mater., 2020, vol. 3, no. 4, p. 3779. https://doi.org/10.1021/acsanm.0c00464
Gabor, V., Kassai, P., and Santos, E.N., Environ. Sci. Pollut. Res., 2020, vol. 27, no. 18, p. 22195. https://doi.org/10.1007/s11356-020-08047-1
Fazullin, D.D. and Mavrin, G.V., Chem. Petrol. Eng., 2020, vol. 56, nos. 3‒4, p. 215. https://doi.org/10.1007/s10556-020-00761-4
Funding
The work was financially supported in the framework of the program for the State support of young Russian PhD scientists (grant no. mk-1107.2019.8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Fazullin, D.D., Fazullina, L.I. & Mavrin, G.V. Purification of Oily Wastewater by a Dynamic Membrane with a Cellulose Acetyl Surface Layer. Russ J Gen Chem 91, 2779–2785 (2021). https://doi.org/10.1134/S107036322113017X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036322113017X