Abstract
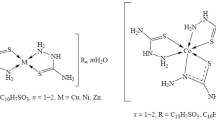
The complexes of 3d-metal 5-sulfosalicylates with phenylacethydrazide are synthesized: [Co(L)3]HSSal · 3H2O (I), [Ni(L)3]HSSal · 3H2O (II), and [Zn(L)3]HSSal · 6H2O (III) (L is phenylacethydrazide, and HSSal2– is 5-sulfosalicylic acid anion). Compounds I–III are characterized by the methods of chemical analysis, IR spectroscopy, diffuse reflectance spectroscopy, luminescence, and thermogravimetry. Compound II is studied by X-ray diffraction analysis (СIF file CCDC no. 1819696). In the complex cation [Ni(L)3]2+, the nickel atom has a face octahedral coordination mode by three O atoms and three N atoms of three bidentate chelate ligands L. The complex cations, HSSal2– anions, and crystallization water molecules are joined by a branched system of hydrogen bonds. Complexes I–III are luminescent, and complex III exhibits the brightest luminescence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Hydrazides of carboxylic acids can manifest various useful biological properties, in particular, bactericidal, fungicidal, antivermicular, and anticonvulsant properties. They are the starting materials for the preparation of a wide range of derivatives used as pharmaceuticals and surfactants [1]. Many metal complexes with hydrazide ligands also manifest biological activity [2]. In turn, 5-sulfosalicylic acid and its metal complexes are also biologically active: antiulcer, antimicrobial, fungicidal, and anti-inflammatory activity [3]. The compounds containing 5-sulfosalicylate anion possess luminescence properties [4–6]. It can be assumed that a combination of such potentially active fragments in one compound can be very efficient. Literature data on the complex formation of 3d metals with phenylacethydrazide (L) are lacking. We have earlier synthesized and structurally characterized the compound of nickel(II) benzoate with phenylacethydrazide: [Ni(L)3](С6Н5)2 · 4H2O [7]. Many structural data are available for 5-sulfosalicylates, which show that their anions in coordination compounds can perform different functions. They can be mono- [6], di- [4, 5], or triply [8] deprotonated outer-sphere anions and can be coordinated via the monodentate mode through the sulfo [5, 9] and carboxy group [10] or via the bidentate mode through the carboxy group and deprotonated phenolic –ОН group [11]. They can be coordinated via the bridging mode through the carboxy and sulfo groups [12] and even simultaneously through all functional groups [13]. We have earlier prepared the compound in which 5-sulfosalicylic acid is present in the undissociated form in the external sphere [14].
In this work, we describe the synthesis, structures, IR spectra, diffuse reflectance and luminescence spectra, and thermogravimetric curves of the aqua complexes of 3d-metal 5-sulfosalicylates with phenylacethydrazide: [М(L)3]HSSal · mH2O (M is cobalt(II), m = 3 (I); M is nickel(II), m = 3 (II); and M is zinc(II), m = 6 (III)). The crystal and molecular structures of compound II (L is phenylacethydrazide, and HSSal2– is 5-sulfosalicylic acid anion) were determined by X-ray diffraction analysis.
EXPERIMENTAL
Synthesis of complexes I–III. 3d-Metal nitrate (0.01 mol) was dissolved in water (40 mL), and NaOH (0.8 g, 0.02 mol) was added to the obtained solution. A formed precipitate of 3d-metal hydroxide was filtered off, washed with water, and transferred to a beaker with 5-sulfosalicylic acid (2.18 g, 0.01 mol). Water was added to the mixture to the complete dissolution of the compounds. The obtained solution was added to a solution (50 mL) of phenylacethydrazide (4.5 g, 0.03 mol) in methanol, and the resulting mixture was left to stay for precipitation. The precipitate formed was filtered off, washed with water, and dried in air to a constant weight. In the case of compound II, the crystals turned out to be suitable for X-ray diffraction analysis.
For C31H40N6O12SCo (I) | |||||
Anal. calcd., % | С, 47.75 | H, 5.13 | N, 10.78 | S, 4.11 | Co, 7.57 |
Found, % | С, 47.57 | H, 5.47 | N, 10.75 | S, 4.13 | Co, 7.17 |
For C31H40N6O12SNi (II) | |||||
Anal. calcd., % | С, 47.75 | H, 5.13 | N, 10.78 | S, 4.11 | Ni, 7.57 |
Found, % | С, 47.78 | H, 5.41 | N, 10.71 | S, 4.39 | Ni, 7.29 |
For C31H46N6O15SZn (III) | |||||
Anal. calcd., % | С, 44.34 | H, 5.48 | N, 10.01 | S, 3.81 | Zn, 7.75 |
Found, % | С, 44.77 | H, 5.54 | N, 10.43 | S, 3.45 | Zn, 7.37 |
Elemental analyses to the metal and sulfur were conducted by atomic emission spectrometry with inductively coupled plasma on a PerkinElmer Optima 8000 instrument. Analyses to carbon, hydrogen, and nitrogen were carried out on a CHN analyzer.
IR spectra were recorded on a PerkinElmer SPECTRUM BX II FT-IR SYSTEM instrument for samples as KBr pellets. Diffuse reflectance spectra were detected on a Lambda-9 spectrophotometer (PerkinElmer) using MgO (βMgO = 100%) as a standard.
Thermogravimetric curves were measured on a Paulik–Paulik–Erdey derivatograph in air with a heating rate of 10 deg/min.
Luminescence spectra were recorded on a Fluorolog FL 3-22 spectrofluorimeter using an ozone-free xenon lamp (450 W) and an R928P photoelectric multiplier (Hamamatsu, Japan) for the UV-visible range. Excitation and luminescence spectra were corrected taking into account the radiation distribution of the xenon lamp and sensitivity of the photoelectric multiplier. The integrated intensity of luminescence (Ilum) was measured from the surface area under the band contour. The luminescence quantum yield (Φ) of the samples was determined by the relative method using the equation
where Φst and Φ are the quantum yields of the luminescent standard and the sample under study, respectively; Rst and Rx are the luminescence reflections of the standard and studied sample, respectively; and Ist and Ix are the integrated luminescence intensities of the standard and studied sample, respectively [15]. Sodium salicylate was used as a standard for which Φst = 60% in a wide range of excitation wavelengths (160–340 nm) [16]. The reflection in percentage was determined according to a described procedure [17] by scanning the luminescence monochromator in a range of 288–292 nm, whereas the excitation monochromator was fixed at λexc = 290 nm. The reflection standard was MgO (Rst = 0.97). The uncertainty of quantum efficiency determination was ±10%.
X-ray diffraction analysis of compound II. The structure was solved by a direct method and refined by least squares in the full-matrix anisotropic approximation for all non-hydrogen atoms. The positions of hydrogen atoms in the organic ligands and in the anion were calculated geometrically and included into refinement by the riding model. Of six hydrogen atoms of three water molecules, only one atom, namely, Н(7w), was localized and refined in the isotropic approximation. One of the phenyl groups, С(20А)–С(24А) atoms, is randomly disordered in two equally probable positions. The high R factor for the structure of compound II is determined by a poor quality of the crystal. The crystallographic data and experimental characteristics for the crystal of compound II are presented in Table 1. Selected bond lengths and bond angles are listed in Table 2. The geometric parameters of intra- and intermolecular bonds are given in Table 3.
The full crystallographic data for compound II were deposited with the Cambridge Crystallographic Data Centre (CIF file СCDC no. 1819696; deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac. uk/data_request/cif).
RESULTS AND DISCUSSION
The chemical analysis showed the ratio М : L = 1 : 3 for compounds I–III.
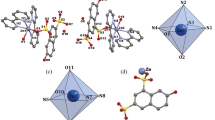
The structure of complex II was determined by X‑ray diffraction analysis. The structural units of the crystal of compound II are complex cations [Ni(L)3]2+, anions HSSal–, and crystallization water molecules in a ratio of 1 : 1 : 3 (Fig. 1). The Ni atom is coordinated at the vertices of the octahedron by three oxygen atoms and three nitrogen atoms of three bidentate chelate (О,N) ligands L. When coordinating with the nickel atom, the phenylacethydrazide ligands (L) close three five-membered metallocycles NiNNCO. The average distances are as follows: Ni–N 2.080 ± 0.007 and Ni–O 2.055 ± 0.011 Å. The average chelate NNiO angle is 79.66° ± 0.57°. The geometric parameters (Ni–N 2.083 ± 0.001, Ni–O 2.053 ± 0.01 Å) and chelate angle NNiO (79.63° ± 0.47°) in the aforementioned compound [Ni(L)3(C6H5)2 · 4H2О [7] almost coincide with similar values in complex II with a similar composition.
Nevertheless, different geometric isomers take place in similar complex cations in the structures of compounds I and IV: cis,trans-meridian (mer) in compound IV and cis,cis-face (fac) with triples of N3 and O3 at the opposite faces of the NiN3O3 octahedron in compound II. The structural units of the crystal of compound II are joined by a branched network of hydrogen bonds О–Н···О and N–H···O involving all hydrogen atoms of the NH2 and NH of molecules L (as mentioned in Experimental, only one hydrogen atom of six atoms of the Н2О molecules is localized): Н···О 1.78–2.30, N···O 2.800–3.026, and O···O 2.475–2.804 Å and angles О(N)–H···O are 129°–180° (Table 3).
A similar stoichiometry and resembling IR spectra (Table 4) and thermogravimetric data (Table 5) assert that the structures of the internal and external spheres for the cobalt (I) and zinc (III) complexes are the same as those for the nickel complex (II). The IR spectra of compounds I–III are presented in Table 4. The absorption bands related to vibrations of phenylacethydrazide were assigned taking into account published data [21–24]. A comparison of the IR spectra of free phenylacethydrazide and complexes I–III shows that the frequency of stretching vibrations of the C=O group (amide I) increases upon complex formation. This band usually shifts to the low-frequency range for the coordination of hydrazides through oxygen [25–31]. The unusual behavior of the band caused by the ν(C=O) vibrations is related to the fact that its frequency is decreased in the spectrum of the initial phenylacethydrazide because of intra- and intermolecular hydrogen bonds. It has been proved as early as in 1956 that phenylacethydrazide both in the solid state and in concentrated chloroform solutions contains hydrogen bonds [22], and the expected frequency of the ν(C=О) band was shown to be ~1710 cm–1. A comparison with ν(C=О) without hydrogen bonds shows that the shift to lower frequencies is observed upon complex formation. Therefore, the frequency of the ν(C=О) band of phenylacethydrazide is decreased because of hydrogen bond formation more significantly than due to complex formation. For ω(NH2) wagging vibrations, the doublet disappears and the frequency increases compared to that of the initial phenylacethydrazide. The ν(С–С) bands of the aromatic ring in the IR spectra of complexes I–III disappear as well as the bands of symmetric vibrations νs(C–H) of the CH2 group. The νas frequency of this group is split into a doublet, and the frequency of the bending δ(СH2) vibrations remains unchanged. The triplet at 1130–1210 cm–1 corresponding to vibrations of various bonds involving the nitrogen atoms becomes doublet in the IR spectra of the complexes. Therefore, the character of changing the absorption bands in the IR spectra of the synthesized complexes compared to the spectrum of free phenylacethydrazide is caused by the participation of the oxygen and nitrogen atoms in the formation of the chelate cycle.
The absorption bands related to the vibrations of the 5-sulfosalicylate anion were assigned taking into account published data [3, 4, 32–34]. The ν(C=O) band, which appears at 1678 cm–1 in the spectrum of free 5-sulfosalicylic acid, disappears in the spectra of complexes I–III. Instead this band, the νas(COO–) and νs(COO–) bands appear, the difference between which (designated as ∆ in Table 4) for all the three complexes lies in a range of 155–159 cm–1. This corresponds to the deprotonation of the carboxy group. In the IR spectra of all complexes, the number of bands corresponding to the ν(SO2) vibrations decreases, which is evidently related to an increase in the symmetry of the sulfo group upon complex formation. This is consistent with the deprotonation of the SO3H group. Thus, the absorption bands in the IR spectra of complexes I–III correspond to the doubly deprotonated form of the sulfur group.
The thermogravimetric curves of compounds I–III are almost similar: two endo-effects corresponding to the water loss and destruction are observed at first, and then several exo-effects evidently corresponding to the burning out of the destruction products appear. For complexes I and II, the mass loss at the first effect agrees well with the loss of crystallization water (in both cases, the theoretical content is 6.9%). Obviously, not all water leaves from compound III at comparatively low temperatures, which is possibly due to its larger amount (and the presence of stronger hydrogen bonds). Depending on the complexing agent, the thermal stability decreases in the order Ni2+ > Co2+ > Zn2+.
The data on the diffuse reflectance spectra (Table 6) for complexes I and II are consistent with their octahedral structure [35].
All synthesized complexes are luminescent (Table 7). Complex III has the brightest luminescence with a maximum at 416 nm, which results in the blue color of the radiation (Fig. 2a, 1). The luminescence excitation spectra (Fig. 2b) are characterized by broad bands in the UV range with maxima at 280–308 nm undergoing the bathochromic shift in the order Zn(II)–Ni(II)–Co(II). For complex III, the position of the maximum (280 nm) coincides with that for sodium salicylate, indicating the determining contribution of the sulfosalicylate anion to the luminescence of the obtained compounds. The quantum yield of complex III measured by the relative method comparing with the luminescence standard (sodium salicylate) was 35.4%.
In complexes II and I, the radiation is considerably quenched, which is related to the energy transfer from the sulfosalicylate ion to the central metal ions. Transition metal ions with the unfilled 3d shell are characterized by a similar quenching of molecular fluorescence [36]. The efficiency of the energy transfer to the central ion-acceptor depends on the degree of overlapping of the fluorescence and absorption bands caused by d–d transitions. In our case, the radiation intensity for complex II exceeds that of compound I by 7.7 times. The luminescence maximum for complex II lies in the range of minimum absorption between the bands of the π–π* transitions of the organic moiety and the band of the 3A2g → 3T1g(P) transition of Ni(II) with a maximum at 576 nm (Fig. 3b).
In the case of complex I, on the contrary, the luminescence band and 4T1g(F) → 4T1g(P) transition of Co(II) with a maximum at 490 nm are significantly overlapped (Fig. 3a). Thus, the luminescence with a quantum yield of 2.4% was detected for complex II, whereas the value obtained for complex I (0.3%) indicates the almost complete luminescence quenching.
REFERENCES
Arjunan, V., Rani, T., Mythili, C.V., and Mohan, S., Spectrochim. Acta, Part A, 2011, vol. 79, no. 3, p. 486.
Ul Ain, Q., Ashiq, U., Ara, J.R., and Mahrooof-Tahir, M., Spectrochim. Acta, Part A, 2013, vol. 115, p. 683.
Fan, S.-R. and Zhu, L.-G., J. Mol. Struct., 2007, vol. 827, nos. 1–3, p. 188.
Mistri, S., Zangrando, E., Farnetti, E., et al., Polyhedron, 2015, vol. 89, p. 250.
Yan, Ch.-F., Jiang, F.-L., Chen, L., et al, J. Solid State Chem., 2009, vol. 182, no. 11, p. 3162.
Lamshöft, M., Storp, J., Ivanova, B., et al., Polyhedron, 2011, vol. 30, no. 15, p. 2564.
Koksharova, T.V., Sergienko, V.S., Surazhskaya, M.D., et al., Russ. J. Inorg. Chem., 2017, vol. 62, no. 12, p. 1568. doi 10.1134/S0036023617120105
Xia, M. and Wei, C.-L., J. Struct. Chem., 2013, vol. 54, no. 1, p. 110.
Gao, Sh., Huo, L.-H., Zhao, H., et al., Acta Crystallogr., Sect E: Struct. Rep. Online, 2005, vol. 61, no. 2, p. m290.
Han, Q., Wang, X.-Ch., Li, X.-Y., et al., Acta Crystallogr., Sect E: Struct. Rep. Online, 2009, vol. 65, no. 11, p. m1282.
Yenikaya, C., Sari, M., Ilkimen, H., et al., Polyhedron, 2011, vol. 30, no. 3, p. 535.
Chen, Zh.-F., Shi, Sh.-M., Hu, R.-X., et al., Chin. J. Chem., 2003, vol. 21, no. 8, p. 1059.
Sun, Y.-Q., Fan, L.-L., Gao, D.-Zh., et al., Dalton Trans., 2010, vol. 39, no. 40, p. 9654.
Antsyshkina, A.S., Koksharova, T.V., Sergienko, V.S., et al., Russ. J. Inorg. Chem., 2014, vol. 59, no. 12, p. 1417. doi 10.1134/S0036023614120031
Lagorio, M.G., Dicelio, L.E., Litter, M.I., et al., J. Chem. Soc., Faraday Trans., 1998, vol. 94, no. 3, p. 419.
Bril, A. and de Jager-Veenis, A.W., J. Electrochem. Soc., 1976, vol. 123, no. 3, p. 396.
Nazarov, M., Noh, D.Y., Sohn, J., et al., J. Solid State Chem., 2007, vol. 180, no. 9, p. 2493.
APEX2, SAINT, SADABS, Madison: Bruker AXS Inc., 2008–2009.
Altomare, A., Gascsrano, G., Giacovazzo, C., and Guagliard, A., J. Appl. Cryst. A, 1993, vol. 26, p. 343.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, vol. 64, p. 112.
Odunola, O.A., Adeoye, I.O., and Woods, J.A.O., Synth. React. Inorg. Metal-Org. Chem., 2002, vol. 32, no. 4, p. 801.
Jensen, J.B., Acta Chem. Scand., 1956, vol. 10, no. 4, p. 667.
Gordon, A. and Ford, R., The Chemist’s Companion: A Handbook of Practical Data, Techniques, and References, New York: Wiley, 1972.
Ul Ain, Q., Ashiq, U., Ara, J.R., et al., Arab. J. Chem., 2017, vol. 1, no. 4, p. 488.
Issa, R.M., El-Shazly, M.F., and Iskander, M.F., Z. Anorg. Allg. Chem., 1967, vol. 354, nos. 1−2, p. 90.
Zidan, A.S.A., Synth. React. Inorg. Metal-Org. Chem., 2004, vol. 34, no. 4, p. 743.
Gogorishvili, P.V., Karkarashvili, M.V., and Kalandarishvili, D.Z., Zh. Neorg. Khim., 1969, vol. 14, no. 6, p. 1516.
Dutta, A.A. and Chaudhuri, N.R., J. Inorg. Nucl. Chem., 1971, vol. 33, no. 1, p. 189.
Odunola, O.A., Adeoye, I.O., Woods, J.A.O., et al., Synth. React. Inorg Metal-Org. Chem., 2003, vol. 33, no. 2, p. 205.
Narang, K.K. and Singh, M., Synth. React. Inorg. Metal-Org. Chem., 1985, vol. 15, no. 6, p. 821.
Narang, K.K., Pandey, J.P., Singh, K.P., et al., Synth. React. Inorg. Metal-Org. Chem., 1990, vol. 20, no. 10, p. 1301.
Fan, S.-R. and Zhu, L.-G., Chin. J. Chem., 2005, vol. 23, no. 10, p. 1292.
Varghese, T.H., Panicker, Y.C., and Philip, D., J. Raman Spectrosc., 2007, vol. 38, no. 3, p. 309.
Nakanishi, K. Infrared Spectra Absorption Spectroscopy, Tokyo: Nankodo Company, 1962.
Lever, A., Inorganic Electronic Spectroscopy, New York: Elesvier, 1984, vol. 2.
Fabbrizzi, L., Licchelli, M., Pallavicini, P., et al., Analyst, 1996, vol. 121, p. 1763.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Koksharova, T.V., Sergienko, V.S., Surazhskaya, M.D. et al. Syntheses and Characterization of Coordination Compounds of 3d-Metal 5-Sulfosalicylates with Phenylacethydrazide: Crystal Structure of [Ni(L)3]HSSal · 3H2O (L is Phenylacethydrazide, and HSSal2– is 5-Sulfosalicylic Acid Anion). Russ J Coord Chem 44, 678–687 (2018). https://doi.org/10.1134/S1070328418110040
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328418110040