Abstract
In order to discover novel high efficiency and low toxic anticancer drugs, a series of novel 2-amino-5-ethylpyrimidine derivatives (XIIa–x) were designed and synthesized. Their antiproliferative activities against four human cancer cells were evaluated by MTT assay. Among the synthetic target compounds, the compound 5-ethyl-6-((4-methoxybenzyl)thio)-N4-(3,4,5-trimethoxyphenyl)pyrimidine-2,4-diamine (XIIw) showed the most potent antiproliferative activity against MGC-803 (human gastric cancer cells), with the IC50 values of 1.02 ± 0.16 μM. Meanwhile, this compound inhibited colony formation of MGC-803 cells. Furthermore, this compound blocked the cell cycle in S phase and induced cell apoptosis. Collectively, our findings indicated that this compound was a valuable lead compound to design new anticancer drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Within a global scale, cancer has destroyed a lot of happiness families, and the number of people far exceeds the sum of human immunodeficiency viruses, tuberculosis and malaria [1]. In recent years, chemotherapy, surgery, radiotherapy and immunotherapy have been the most common types of cancer treatment today [2, 3]. Although there are several different categories of anticancer drugs can be used in clinical practice, some tricky problems still need to be solved, such as side effects and inefficiency. Therefore, discovery and development of more effective and low toxic anticancer drugs remain urgent [4, 5].
Pyrimidine is nitrogen-containing heterocycle with a wide range of biological activity, including antitumor, anti-HIV, antibacterial, etc. [6–10]. Today, many pyrimidine-based compounds have been applied to clinical practice by many countries, such as Imatinib (I), Dasatinib (II) and Brigatinib (III) [11–15] (Fig. 1), which makes it become a basic scaffold for the design and development of new drugs. Meanwhile, compounds GNE-6640 and GNE-6776 were selected as the lead compounds (Fig. 2), according to the literature, the amino and ethyl group of the lead compounds, which played important roles in antiproliferative activity [16, 17]. Based on scaffold hopping approach through changing the core structure, we changed the pyridine ring to a pyrimidine ring and retained the amino and ethyl group of the lead compounds.
In addition, 4-methoxybenzyl has a very wide range of inhibitory activity for cancer, it has been reported that compounds (IV–VI) [18–20] have good inhibitory effect on tumor cells, and they all contain 4-methoxybenzyl group. Therefore, encouraged by the above research, a series of 2-amino-5-ethylpyrimidine derivatives containing 4-methoxybenzyl by using the combination principles were synthesized and the antiproliferative activity of target compounds was evaluated in vitro by methyl thiazolyl tetrazolium (MTT) assay.
RESULTS AND DISCUSSION
Chemistry
The synthesis method of preparing compounds is set forth in Scheme 1. Firstly, taking commercially available diethyl ethylmalonate as starting material, compound (IX) was synthesized by stirring with guanidine hydrochloride at 80°C for 6 h. Then, compound (X) was obtained from the reaction of compound (IX) with phosphorus oxychloride at 90°C for 6 h. Next, compound (X) was reacted with differently substituted anilines to obtain compounds (XIa–x) in ethanol at 100°C for 2 h. Finally, compounds (XIIa–x) were synthesized using compounds (ZhengzhouXia-x) with 4‑methoxybenzyl mercaptan under basic condition in N,N-dimethylformamide at 80°C for 1 h. Meanwhile,the structures of target compounds were proved by 1H NMR, 13C NMR and HRMS.
Scheme 1 . Reagents and conditions: (i) CH3ONa, CH3OH, 80°C, 6 h; (ii) POCl3, 90°C, 6 h; (iii) different anilines, ethanol, 100°C, 2 h; (iv) DMF, KOH, 80°C, 1 h.
Evaluation of Biological Activity
Antitumor activity. The antiproliferative activities of these target compounds were assessed by MTT assay to four human tumor cell lines, including human prostate cancer cells (PC-3), human gastric cancer cells (MGC-803), human breast cancer cells (MCF-7) and human non-small cell lung cancer (H1975). 5‑Fluorouracil was used as positive control. The experimental results were summarized in Table 1.
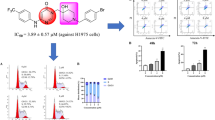
As shown in Table 1, most compounds exhibit a moderate antiproliferative activity on four cell lines. Comparing compound (XIIa) with other compounds, it could be found that different substituents on aniline have a certain effect on bioactivity. Compared with compounds (XIIb–d), (XIIe–g) and (XIIh–j), the contribution to enhance antiproliferative activity was F > Cl > Br. In addition, it could be seen that the antiproliferative activity of the trifluoromethyl substituted compounds (XIIk) and (XIIl) were generally better than other electron withdrawing substitutions. From the biological data of compounds (XIIm–o), (XIIp–r) and (XIIw), we could know that as the electron donating ability of the substituent increasing, the antiproliferative activity increased, and the order of bioactivity was meta-substitution > para-substitution > ortho-substitution. Among them, compound (XIIw) with more electron-donating groups had the best antiproliferative activity, against MGC-803 cell lines with the IC50 values of 1.02 ± 0.16 μM, which was superior to the positive control 5-Fu (IC50 = 9.05 μM). As a consequence, in order to study the mechanism of target compounds, the compound (XIIw) and human gastric cancer cells (MGC-803) were selected to perform a more deep level of experiment.
Compound (XIIw) inhibited the proliferation of MGC-803 cells. Firstly, we studied the effects of compound (XIIw) on the cell proliferation by Plate cloning test for 0, 0.5, 1 and 2 μM respectively. As displayed in Fig. 3, compound (XIIw) could significantly and dose-dependently reduced the formation of colonies in MGC-803 cells compared with the control group. Most antitumor compounds inhibit cell proliferation through induction of cell cycle arrest [21]. In order to better explain the antiproliferative activity of MGC-803 cells, we conducted a cell-cycle analysis. After treatment with compound (XIIw) 0, 0.5, 1 and 2 μM for 24 h, the proportion of MGC-803 cells in S phase increased from 36.11 to 63.73%, which indicated that compound (XIIw) suppressed the reproduction of MGC-803 cells by blocking the cell cycle in S phase (Fig. 4).
Compound (XIIw) induced MGC-803 cells apoptosis. Apoptosis is considered to be an important way to kill cancer cells in most anti-tumor drugs. As a consequence, by using DAPI staining, we explored the impact of compound (XIIw) on apoptosis of MGC-803 cells. As we observed in Fig. 5, after 48 h with a designated concentration of compound (XIIw), the number of changes in apoptotic morphology increased significantly, which indicating that compound (XIIw) could induce apoptosis. Then, we performed a biparametric cytofluorimetric analysis to better characterize the compound (XIIw) induced apoptosis pattern. After MGC-803 cells were treated with different concentration of compound (XIIw) for 48h, the proportion of apoptotic cells in MGC-803 cells increased from 9.59 to 25.98%, which suggested that compound (XIIw) dose-dependently induced the cellular apoptosis (Fig. 6).
Effect of compound (XIIw) against human normal cell line. As shown in Table 2, we found compound (XIIw) showed weaker cytotoxicity against GES-1 (human normal esophageal cell) compared with 5‑Fluorouracil (5-Fu). Meanwhile, the SI (selectivity index) value of compound (XIIw) was approximately 27 times than the positive control 5-Fu.
EXPERIMENTAL
Materials
Reagents and solvents were purchased from a regular platform without purification. Silica gel: China Qingdao Ocean Chemical Group Corporation (200−300 mesh silica gel). Melting points were determined on an X-5 micro melting apparatus and are uncorrected. 1H NMR and 13C NMR spectra were measured on a Bruker 400 and 101 MHz spectromerer respectively, using TMS as an internal standard. High-resolution mass spectrometry (HRMS) were recorded on the Water segment Q–T of the micrometer by electrospray ionizaton (ESI).
MTT Assay
By using MTT method to measure compound activity, human cancer cells in logarithmic growth phase were first inoculated with 3000–5000 cells/well in 96-well plates. After 24 h of cell culture, the cells were then treated in triplicated with various compounds in different concentrations at 37°C in a moist 5% CO2 atmosphere for 72 h. After that, MTT was added into each well with a final concentration of 5 mg/mL. After 4 hours of culture at 37°C, the medium was sucked out. Then add 150 μL DMSO to each well to dissolve the formazan and shake the plates on a shaker for 10 min. OD values were measured at 490 nm using a microplate reader, and IC50 values were calculated using SPSS 26.0 software. The result is the mean ± standard deviation of three independent experiments.
Colony Formation Assaysis
MGC-803 cells in logarithmic growth phase were inoculated into 6-well plates with 500 cells/well. After 24 h culture, the cells were treated with compound (XIIw) with different concentration gradients (0, 0.5, 1 and 2 μM). The cells were continued to be cultured at 37°C and 5% CO2 until visible form was formed at the bottom of 6-well plates. When the cells of suitable size were collected, the culture was terminated and the cells were stained with crystal violet dye. After the film dries naturally, shoot with a camera.
Cell Cycle Analysis
MGC-803 cells in logarithmic growth phase were inoculated into 6-well plates with 3 × 105 cells per well. After cell adherence, compound (XIIw) with different concentrations (0, 0.5, 1 and 2μM) was added respectively for 24 h, then the cells were fixed with 75% ethanol and incubated with propidium iodide (PI) dye at 37°C for 30 min under dark conditions. Flow cytometry was used for detection.
Cell Morphology Analysis and DAPI Staining
MGC-803 cells at logarithmic growth stage were digested, centrifuged, and counted, then inoculated into 6-well plates with 2 × 105 cells/well. After cell adherence, the cells were treated with compound (XIIw) of different concentrations (0, 1 and 2 μM) for 48 h. The cells were incubated with DAPI solution, observed and photographed under fluorescence microscope.
Apoptosis Assaysis
MGC-803 cells in logarithmic growth phase were inoculated with 3 × 105 cells/well in 6-well plates. After cell adherence, compound (XIIw) was added with different concentrations (0, 0.5, 1 and 2 μM) for 48 h. Annexin V-FITC/PI (Keygen Biotech, China) staining solution was used to incubate the cells, and cell apoptosis was detected by flow cytometry.
Chemistry
Synthesis of 2-amino-5-ethylpyrimidine-4,6-diol (IX), 4,6-dichloro-5-ethylpyrimidin-2-amine (X) and compounds (XIa–x) were synthesized according to the literatures [22–24].
General procedure for synthesis of 2-amino-5-ethylpyrimidine derivatives (XIIa–x). Compound (XIa–x) (0.80 mmol) was added in 5 mL of N,N-dimethylformamide, then KOH (1.21 mmol) was added. Next, 4‑methoxybenzyl mercaptan (0.96 mmol) was added dropwise to the above system and the reaction system was heated to 80°C for 1 h. After completion of the reaction (TLC detection), filtered, dried. Finally, Purified the crude product by column chromatography to obtain compounds (XIIa–x).
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-phenylpyrimidine-2,4-diamine (XIIa) white solid, yield 79.49%; mp 148.1–148.9°C; 1H NMR (400 MHz, DMSO-d6) δ 7.94 (s, 1H, NH), 7.73–7.65 (m, 2H, Ar-H), 7.33 (d, J = 8.6 Hz, 2H, Ar-H), 7.25 (dd, J = 8.5, 7.3 Hz, 2H, Ar-H), 7.00–6.92 (m, 1H, Ar-H), 6.84 (d, J = 8.6 Hz, 2H, Ar-H), 6.07 (s, 2H, NH2), 4.31 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.54 (t, J = 7.4 Hz, 2H, CH2CH3), 1.00 (t, J = 7.3 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.6, 159.8, 158.1, 157.6, 140.6, 130.7, 130.2, 128.1, 121.9, 121.5, 113.7, 104.7 (C pyrimidine and benzene), 55.0 (OCH3), 32.0 (SCH2), 18.0 (CH2CH3), 12.9 (CH2CH3). HR-MS (ESI): Calcd. for C20H23N4OS [M + H]+: 367.1593, found: 367.1592.
5-Ethyl-N4-(4-fluorophenyl)-6-((4-methoxybenzyl)-thio)pyrimidine-2,4-diamine (XIIb) white solid, yield 57.98%; mp 150.5–151.3°C; 1H NMR (400 MHz, DMSO-d6) δ 8.00 (s, 1H, NH), 7.71–7.66 (m, 2H, Ar-H), 7.35–7.31 (m, 2H, Ar-H), 7.08 (t, J = 8.9 Hz, 2H, Ar-H), 6.86–6.82 (m, 2H, Ar-H), 6.06 (s, 2H, NH2), 4.31 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.50 (dt, J = 3.8, 1.9 Hz, 2H, CH2CH3), 1.00 (t, J = 7.3 Hz, 3H, CH2CH3).13C NMR (101 MHz, DMSO-d6) δ 168.8, 165.1, 164.0, 163.4, 162.8, 142.1, 135.9, 135.4, 128.7, 119.8, 118.9, 109.8 (C pyrimidine and benzene), 60.2 (OCH3), 37.2 (SCH2), 23.2 (CH2CH3), 18.1 (CH2CH3). HR-MS (ESI): Calcd. for C20H22FN4OS [M + H]+: 385.1498, found: 385.1498.
5-Ethyl-N4-(3-fluorophenyl)-6-((4-methoxybenzyl)-thio)pyrimidine-2,4-diamine (XIIc) white solid, yield 47.57%; mp 139.2–139.9°C; 1H NMR (400 MHz, DMSO-d6) δ 8.08 (s, 1H, NH), 7.84–7.76 (m, 1H, Ar-H), 7.52 (dd, J = 8.2, 2.2 Hz, 1H, Ar-H), 7.37–7.31 (m, 2H, Ar-H), 7.25 (td, J = 8.2, 7.0 Hz, 1H, Ar-H), 6.87–6.81 (m, 2H, Ar-H), 6.74 (td, J = 8.4, 2.5 Hz, 1H, Ar-H), 6.21 (s, 2H, NH2), 4.32 (d, J = 2.2 Hz, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.55 (d, J = 7.4 Hz, 2H, CH2CH3), 1.00 (td, J = 7.1, 6.0, 3.5 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 164.2, 163.2, 159.8, 158.2, 157.2, 142.7, 130.5, 130.2, 129.4, 116.5, 113.7, 107.9, 107.6, 105.1 (C pyrimidine and benzene), 55.0 (OCH3), 32.0 (SCH2), 17.8 (CH2CH3), 13.0 (CH2CH3). HR-MS (ESI): Calcd. for C20H22FN4OS [M + H]+: 385.1498, found: 385.1499.
5-Ethyl-N4-(2-fluorophenyl)-6-((4-methoxybenzyl)-thio)pyrimidine-2,4-diamine (XIId) white solid, yield 33.89%; mp 122.3–123.1°C; 1H NMR (400 MHz, DMSO-d6) δ 7.82 (s, 1H, NH), 7.57–7.54 (m, 1H, Ar-H), 7.33 (d, J = 8.6 Hz, 2H, Ar-H), 7.23–7.17 (m, 1H, Ar-H), 7.14 (td, J = 7.0, 3.4 Hz, 2H, Ar-H), 6.84 (d, J = 8.6 Hz, 2H, Ar-H), 5.97 (s, 2H, NH2), 4.31 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.51 (dd, J = 8.4, 5.7 Hz, 2H, CH2CH3), 1.03 (t, J = 7.3 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.4, 160.1, 158.3, 158.1, 157.5, 130.7, 130.2, 128.0, 127.6, 125.4, 124.0, 115.5, 113.7, 104.4 (C pyrimidine and benzene), 55.0 (OCH3), 31.9 (SCH2), 18.1 (CH2CH3), 12.6 (CH2CH3). HR-MS (ESI): Calcd. for C20H22FN4OS [M + H]+: 385.1498, found: 385.1499.
N4-(4-Chlorophenyl)-5-ethyl-6-((4-methoxybenzyl)-thio)pyrimidine-2,4-diamine (XIIe) white solid, yield 62.89%; mp 138.7–139.5°C; 1H NMR (400 MHz, DMSO-d6) δ 8.08 (s, 1H, NH), 7.82–7.74 (m, 2H, Ar-H ), 7.34 (d, J = 8.6 Hz, 2H, Ar-H), 7.30–7.25 (m, 2H, Ar-H), 6.85 (t, J = 5.8 Hz, 2H, Ar-H), 6.14 (s, 2H, NH2), 4.32 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.58–2.51 (m, 2H, CH2CH3), 1.00 (t, J = 7.3 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 164.0, 159.8, 158.1, 157.3, 139.7, 130.6, 130.2, 127.8, 125.3, 122.8, 113.7, 104.9 (C pyrimidine and benzene), 55.0 (OCH3), 32.0 (SCH2), 17.9 (CH2CH3), 12.9 (CH2CH3). HR-MS (ESI): Calcd. for C20H22ClN4OS [M + H]+: 401.1203, found: 401.1204.
N4-(3-Chlorophenyl)-5-ethyl-6-((4-methoxybenzyl)-thio)pyrimidine-2,4-diamine (XIIf) white solid, yield 62.59%; mp 132.5–133.3°C; 1H NMR (400 MHz, DMSO-d6) δ 8.09 (s, 1H, NH), 7.83 (t, J = 2.0 Hz, 1H, Ar-H), 7.81–7.76 (m, 1H, Ar-H), 7.34 (d, J = 8.6 Hz, 2H, Ar-H), 7.26 (t, J = 8.1 Hz, 1H, Ar-H), 6.98 (dd, J = 7.9, 1.3 Hz, 1H, Ar-H), 6.85 (t, J = 5.8 Hz, 2H, Ar-H), 6.20 (s, 2H, NH2), 4.32 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.58–2.52 (m, 2H, CH2CH3), 1.00 (t, J = 7.3 Hz, 3H,CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 164.2, 159.8, 158.2, 157.2, 142.3, 132.5, 130.5, 130.2, 129.6, 121.2, 120.3, 119.3, 113.7, 105.1 (C pyrimidine and benzene), 55.0 (OCH3), 32.0 (SCH2), 17.9 (CH2CH3), 13.0 (CH2CH3). HR-MS (ESI): Calcd. for C20H22ClN4OS [M + H]+: 401.1203, found: 401.1201.
N4-(2-Chlorophenyl)-5-ethyl-6-((4-methoxybenzyl)-thio)pyrimidine-2,4-diamine (XIIg) white solid, yield 55.12%; mp 139.2–140.1°C; 1H NMR (400 MHz, DMSO-d6) δ 7.81 (dd, J = 8.0, 1.4 Hz, 1H, NH), 7.72 (s, 1H, Ar-H), 7.48 (dd, J = 8.0, 1.3 Hz, 1H,Ar-H), 7.34 (d, J = 8.6 Hz, 2H, Ar-H), 7.30 (dd, J = 7.8, 1.1 Hz, 1H, Ar-H), 7.14 (td, J = 7.8, 1.5 Hz, 1H, Ar-H), 6.85 (d, J = 8.6 Hz, 2H, Ar-H), 6.05 (s, 2H, NH2), 4.31 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.55–2.50 (m, 2H, CH2CH3), 1.06 (t, J = 7.4 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.6, 160.0, 158.1, 158.0, 137.0, 130.6, 130.2, 129.2, 128.2, 127.3, 127.2, 125.3, 113.7, 104.3 (C pyrimidine and benzene), 55.0 (OCH3), 31.9 (SCH2), 18.2 (CH2CH3), 12.5 (CH2CH3). HR-MS (ESI): Calcd. for C20H22ClN4OS [M + H]+: 401.1203, found: 401.1203.
N4-(4-Bromophenyl)-5-ethyl-6-((4-methoxybenzyl)-thio)pyrimidine-2,4-diamine (XIIh) white solid, yield 65.68%; mp 135.2–136.1°C; 1H NMR (400 MHz, DMSO-d6) δ 8.07 (s, 1H, NH), 7.74–7.69 (m, 2H, Ar-H), 7.41 (t, J = 6.0 Hz, 2H, Ar-H), 7.33 (d, J = 8.6 Hz, 2H, Ar-H), 6.85 (t, J = 5.8 Hz, 2H, Ar-H), 6.14 (s, 2H, NH2), 4.31 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.57–2.52 (m, 2H, CH2CH3), 1.00 (t, J = 7.3 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 164.0, 159.8, 158.1, 157.3, 140.1, 130.7, 130.6, 130.2, 123.3, 113.7, 113.3, 104.9 (C pyrimidine and benzene), 55.0 (OCH3), 32.0 (SCH2), 17.9 (CH2CH3), 12.9 (CH2CH3). HR-MS (ESI): Calcd. for C20H22BrN4OS [M + H]+: 445.0698, found: 445.0698.
N4-(3-Bromophenyl)-5-ethyl-6-((4-methoxybenzyl)-thio)pyrimidine-2,4-diamine (XIIi) yellow solid, yield 54.98%; mp 134.5–135.6°C; 1H NMR (400 MHz, DMSO-d6) δ 8.08 (s, 1H, NH), 7.90 (t, J = 1.9 Hz, 1H, Ar-H), 7.88 (d, J = 8.2 Hz, 1H, Ar-H), 7.34 (d, J = 8.7 Hz, 2H, Ar-H), 7.20 (t, J = 8.0 Hz, 1H, Ar-H), 7.12 (dd, J = 7.9, 0.8 Hz, 1H, Ar-H), 6.87–6.82 (m, 2H, Ar-H), 6.20 (s, 2H, NH2), 4.32 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.57–2.51 (m, 2H, CH2CH3), 1.00 (t, J = 7.3 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 164.2, 159.8, 158.1, 157.2, 142.5, 130.5, 130.2, 130.0, 124.1, 123.1, 121.0, 119.8, 113.7, 105.1 (C pyrimidine and benzene), 55.0 (OCH3), 32.0 (SCH2), 17.9 (CH2CH3), 13.0 (CH2CH3). HR-MS (ESI): Calcd. for C20H22BrN4OS [M + H]+: 445.0698, found: 445.0698.
N4-(2-Bromophenyl)-5-ethyl-6-((4-methoxybenzyl)-thio)pyrimidine-2,4-diamine (XIIj) white solid, yield 57.56%; mp 132.5–133.4°C; 1H NMR (400 MHz, DMSO-d6) δ 7.84 (dd, J = 8.0, 1.3 Hz, 1H, NH), 7.69 (s, 1H, Ar-H), 7.64 (dd, J = 8.0, 1.2 Hz, 1H, Ar-H), 7.36 (dd, J = 12.5, 4.9 Hz, 3H, Ar-H), 7.07 (td, J = 7.9, 1.5 Hz, 1H, Ar-H), 6.85 (d, J = 8.6 Hz, 2H, Ar-H), 6.05 (s, 2H, NH2), 4.31 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.55–2.50 (m, 2H, CH2CH3), 1.08 (t, J = 7.3 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.6, 160.0, 158.1, 158.0, 138.2, 132.3, 130.6, 130.2, 127.9, 127.3, 125.7, 119.2, 113.7, 104.2 (C pyrimidine and benzene), 55.0 (OCH3), 31.9 (SCH2), 18.3 (CH2CH3), 12.6 (CH2CH3). HR-MS (ESI): Calcd. for C20H22BrN4OS [M + H]+: 445.0698, found: 445.0696.
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-(4-(trifluoromethyl)phenyl)pyrimidine-2,4-diamine (XIIk) white solid, yield 72.26%; mp 131.4–132.1°C; 1H NMR (400 MHz, DMSO-d6) δ 8.32 (s, 1H, NH), 7.98 (d, J = 8.6 Hz, 2H, Ar-H), 7.57 (d, J = 8.7 Hz, 2H, Ar-H), 7.35 (d, J = 8.7 Hz, 2H, Ar-H), 6.89–6.82 (m, 2H, Ar-H), 6.24 (s, 2H, NH2), 4.33 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.57 (q, J = 7.2 Hz, 2H, CH2CH3), 1.01 (t, J = 7.3 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 164.7, 159.8, 158.2, 157.1, 144.6, 130.5, 130.2, 125.2, 123.3, 121.5, 120.5, 113.7, 105.5 (C pyrimidine, benzene and CF3), 55.0 (OCH3), 32.1 (SCH2), 17.9 (CH2CH3), 13.0 (CH2CH3). HR-MS (ESI): Calcd. for C21H22F3N4OS [M + H]+: 435.1466, found: 435.1465.
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-(3-(trifluoromethyl)phenyl)pyrimidine-2,4-diamine (XIIIl) yellow solid, yield 61.68%; mp 137.3–138.2°C; 1H NMR (400 MHz, DMSO-d6) δ 8.29–8.20 (m, 2H, Ar-H ), 7.98 (s, 1H, NH), 7.47 (t, J = 8.0 Hz, 1H, Ar-H), 7.35 (d, J = 8.2 Hz, 2H, Ar-H), 7.27 (d, J = 7.6 Hz, 1H, Ar-H), 6.85 (d, J = 8.3 Hz, 2H, Ar-H), 6.19 (s, 2H, NH2), 4.33 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.61–2.52 (m, 2H, CH2CH3), 1.02 (t, J = 7.1 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 164.4, 159.7, 158.2, 157.2, 141.5, 130.5, 130.2, 129.1, 128.8, 125.7, 124.5, 117.7, 117.1, 113.7, 105.1 (C pyrimidine, benzene and CF3), 55.0 (OCH3), 32.0 (SCH2), 17.9 (CH2CH3),13.0(CH2CH3). HR-MS (ESI): Calcd. for C21H22F3N4OS [M + H]+: 435.1466, found: 435.1465.
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-(p-tolyl)-pyrimidine-2,4-diamine (XIIm) white solid, yield 54.83%; mp 126.6–127.4°C; 1H NMR (400 MHz, DMSO-d6) δ 7.88 (s, 1H, NH), 7.54 (d, J = 8.0 Hz, 2H, Ar-H), 7.33 (d, J = 8.2 Hz, 2H, Ar-H), 7.06 (d, J = 8.0 Hz, 2H, Ar-H), 6.84 (d, J = 8.3 Hz, 2H, Ar-H), 6.01 (s, 2H, NH2), 4.31 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.54–2.49 (m, 2H, CH2CH3), 2.25 (s, 3H, Ar-CH3), 1.00 (t, J = 7.2 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.3, 159.9, 158.1, 157.8, 138.0, 130.9, 130.7, 130.2, 128.5, 121.9, 113.7, 104.5 (C pyrimidine and benzene), 55.0 (OCH3), 31.9 (SCH2), 20.4 (Ar-CH3), 18.0 (CH2CH3), 12.9 (CH2CH3). HR-MS (ESI): Calcd. for C21H25N4OS [M + H]+: 381.1749, found: 381.1747.
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-(m-tolyl)-pyrimidine-2,4-diamine (XIIn) yellow solid, yield 68.28%; mp 114.3–115.2°C; 1H NMR (400 MHz, DMSO-d6) δ 7.87 (s, 1H, NH), 7.56 (d, J = 8.1 Hz, 1H, Ar-H), 7.47 (s, 1H, Ar-H), 7.33 (d, J = 8.2 Hz, 2H, Ar-H), 7.13 (t, J = 7.8 Hz, 1H, Ar-H), 6.84 (d, J = 8.3 Hz, 2H, Ar-H), 6.78 (d, J = 7.4 Hz, 1H, Ar-H), 6.07 (s, 2H, NH2), 4.31 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.56–2.49 (m, 2H, CH2CH3), 2.28 (s, 3H, Ar-CH3), 1.00 (t, J = 7.2 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.5, 159.9, 158.1, 157.7, 140.5, 137.1, 130.7, 130.2, 127.9, 122.6, 122.1, 118.7, 113.7, 104.8 (C pyrimidine and benzene), 55.0 (OCH3), 32.0 (SCH2), 21.1 (Ar-CH3), 18.0 (CH2CH3), 12.9 (CH2CH3). HR-MS (ESI): Calcd. for C21H25N4OS [M + H]+: 381.1749, found: 381.1748.
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-(o-tolyl)-pyrimidine-2,4-diamine (XIIo) white solid, yield 46.55%; mp 135.1–136.3°C; 1H NMR (400 MHz, DMSO-d6) δ 7.70 (s, 1H, NH), 7.33 (d, J = 8.1 Hz, 2H, Ar-H), 7.25 (d, J = 7.7 Hz, 1H, Ar-H), 7.20 (d, J = 7.3 Hz, 1H, Ar-H), 7.15 (t, J = 7.4 Hz, 1H, Ar-H), 7.07 (t, J = 7.3 Hz, 1H, Ar-H), 6.84 (d, J = 8.1 Hz, 2H, Ar-H), 5.83 (s, 2H, NH2), 4.30 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.56–2.49 (m, 2H, CH2CH3), 2.15 (s, 3H, Ar-CH3), 1.04 (t, J = 7.1 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 162.7, 160.2, 158.8, 158.1, 138.5, 134.2, 130.8, 130.2, 130.1, 127.2, 125.8, 124.9, 113.7, 104.0 (C pyrimidine and benzene), 55.0 (OCH3), 31.9 (SCH2), 18.2 (CH2CH3), 18.2 (Ar-CH3), 12.7 (CH2CH3). HR-MS (ESI): Calcd. for C21H25N4OS [M + H]+: 381.1749, found: 381.1750.
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-(4-methoxyphenyl)pyrimidine-2,4-diamine (XIIp) white solid, yield 62.56%; mp 169.5–170.1°C; 1H NMR (400 MHz, DMSO-d6) δ 7.87 (s, 1H, NH), 7.52 (d, J = 8.7 Hz, 2H, Ar-H), 7.33 (d, J = 8.3 Hz, 2H, Ar-H), 6.85 (s, 2H, Ar-H), 6.83 (s, 2H, Ar-H), 5.96 (s, 2H, NH2), 4.30 (s, 2H, SCH2), 3.72 (s, 3H, NH-Ar-OCH3), 3.72 (s, 3H, CH2-Ar-OCH3), 2.51 (d, J = 6.4 Hz, 2H, CH2CH3), 1.00 (t, J = 7.1 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 162.9, 159.8, 158.0, 157.9, 154.7, 133.4, 130.7, 130.1, 123.7, 113.6, 113.2, 104.2 (C pyrimidine and benzene), 55.1 (NH-Ar-OCH3), 54.9 (CH2-Ar-OCH3), 31.8 (SCH2), 17.9 (CH2CH3), 12.8 (CH2CH3). HR-MS (ESI): Calcd. for C21H25N4O2S [M + H]+: 397.1698, found: 397.1698.
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-(3-methoxy-phenyl)pyrimidine-2,4-diamine (XIIq) yellow solid, yield 65.37%; mp 96.5–97.1°C; 1H NMR (400 MHz, DMSO-d6) δ 7.91 (s, 1H, NH), 7.40 (s, 1H, Ar-H), 7.33 (t, J = 7.4 Hz, 3H, Ar-H), 7.14 (t, J = 8.1 Hz, 1H, Ar-H), 6.85 (d, J = 8.3 Hz, 2H, Ar-H), 6.53 (d, J = 8.1 Hz, 1H, Ar-H), 6.11 (s, 2H, NH2), 4.32 (s, 2H, SCH2), 3.75 (s, 3H, CH2-Ar-OCH3), 3.72 (s, 3H, NH-Ar-OCH3), 2.54 (d, J = 7.2 Hz, 2H, CH2CH3), 1.00 (t, J = 7.1 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.7, 159.8, 159.2, 158.1, 157.6, 141.8, 130.7, 130.2, 128.7, 113.7, 113.5, 107.6, 106.8, 104.9 (C pyrimidine and benzene), 55.0 (CH2-Ar-OCH3), 54.9 (NH-Ar-OCH3), 32.0 (SCH2), 18.0 (CH2CH3), 12.9 (CH2CH3). HR-MS (ESI): Calcd. for C21H25N4O2S [M + H]+: 397.1698, found: 397.1697.
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-(2-methoxy-phenyl)pyrimidine-2,4-diamine (XIIr) white solid, yield 48.94%; mp 94.1–95.2°C; 1H NMR (400 MHz, DMSO-d6) δ 8.29 (d, J = 7.9 Hz, 1H, NH), 7.34 (d, J = 8.9 Hz, 3H, Ar-H), 7.00 (q, J = 8.0 Hz, 2H, Ar-H), 6.91 (t, J = 7.3 Hz, 1H, Ar-H), 6.84 (d, J = 8.2 Hz, 2H, Ar-H), 6.18 (s, 2H, NH2), 4.32 (s, 2H, SCH2), 3.85 (s, 3H, NH-Ar-OCH3), 3.72 (s, 3H, CH2-Ar-OCH3), 2.51–2.46 (m, 2H, CH2CH3), 1.06 (t, J = 7.2 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.2, 160.1, 158.2, 157.5, 149.4, 130.6, 130.2, 129.1, 122.5, 121.5, 120.4, 113.7, 110.7, 104.3 (C pyrimidine and benzene), 56.0 (NH-Ar-OCH3), 55.0 (CH2-Ar-OCH3), 32.0 (SCH2), 18.4 (CH2CH3), 12.2 (CH2CH3). HR-MS (ESI): Calcd. for C21H25N4O2S [M + H]+: 397.1698, found: 397.1697.
N4-(3-Chloro-4-fluorophenyl)-5-ethyl-6-((4-methoxy-benzyl)thio)pyrimidine-2,4-diamine (XIIs) yellow solid, yield 70.86%; mp 112.4–113.1°C; 1H NMR (400 MHz, DMSO-d6) δ 8.11 (s, 1H, NH), 7.96 (d, J = 6.7 Hz, 1H, Ar-H), 7.76 (dd, J = 5.5, 3.4 Hz, 1H, Ar-H), 7.34 (d, J = 8.2 Hz, 2H, Ar-H), 7.28 (t, J = 9.1 Hz, 1H, Ar-H), 6.85 (d, J = 8.3 Hz, 2H, Ar-H), 6.20 (s, 2H, NH2), 4.32 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.52 (d, J = 9.0 Hz, 2H, CH2CH3), 1.00 (t, J = 7.1 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 164.1, 159.8, 158.1, 157.2, 151.1, 138.0, 130.5, 130.2, 122.4, 121.4, 118.5, 116.1, 113.7, 104.7 (C pyrimidine and benzene), 55.0 (OCH3), 32.0(SCH2), 17.9 (CH2CH3), 12.9 (CH2CH3). HR-MS (ESI): Calcd. for C20H21ClFN4OS [M + H]+: 419.1109, found: 419.1108.
N4-(2,4-Dichlorophenyl)-5-ethyl-6-((4-methoxybenzyl)thio)pyrimidine-2,4-diamine (XIIt) white solid, yield 72.26%; mp 131.3–132.0°C; 1H NMR (400 MHz, DMSO-d6) δ 7.79 (s, 1H, NH), 7.76 (s, 1H, Ar-H), 7.63 (s, 1H, Ar-H), 7.37 (d, J = 8.8 Hz, 1H, Ar-H), 7.34 (d, J = 8.3 Hz, 2H, Ar-H), 6.84 (d, J = 8.3 Hz, 2H, Ar-H), 6.06 (s, 2H, NH2), 4.31 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.50 (s, 2H, CH2CH3), 1.06 (t, J = 7.2 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.8, 160.0, 158.2, 157.9, 136.3, 130.6, 130.2, 129.5, 128.6, 128.6, 128.5, 127.3, 113.7, 104.4 (C pyrimidine and benzene), 55.0 (OCH3), 31.9 (SCH2), 18.2 (CH2CH3), 12.5 (CH2CH3). HR-MS (ESI): Calcd. for C20H21Cl2N4OS [M + H]+: 435.0813, found: 435.0814.
N4-(3,4-Dichlorophenyl)-5-ethyl-6-((4-methoxybenzyl)thio)pyrimidine-2,4-diamine (XIIu) white solid, yield 75.54%; mp 129.5–130.1°C; 1H NMR (400 MHz, DMSO-d6) δ 8.18 (s, 1H, NH), 8.06 (s, 1H, Ar-H), 7.85 (d, J = 8.8 Hz, 1H, Ar-H), 7.46 (d, J = 8.9 Hz, 1H, Ar-H), 7.34 (d, J = 8.3 Hz, 2H, Ar-H), 6.85 (d, J = 8.4 Hz, 2H, Ar-H), 6.25 (s, 2H, NH2), 4.32 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.57–2.51 (m, 2H, CH2CH3), 1.00 (t, J = 7.2 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 164.5, 159.7, 158.2, 157.0, 141.0, 130.5, 130.3, 130.2, 129.7, 122.8, 121.9, 120.9, 113.7, 105.2 (C pyrimidine and benzene), 55.0 (OCH3), 32.0 (SCH2), 17.9 (CH2CH3), 13.0 (CH2CH3). HR-MS (ESI): Calcd. for C20H21Cl2N4OS [M + H]+: 435.0813, found: 435.0813.
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-(6-methoxy-pyridin-3-yl)pyrimidi ne-2,4-diamine (XIIv) white solid, yield 78.98%, mp 135.1–135.8°C; 1H NMR (400 MHz, DMSO-d6) δ 8.44 (s, 1H, NH), 8.01 (s, 1H, Ar-H), 7.91 (d, J = 8.8 Hz, 1H, Ar-H), 7.33 (d, J = 8.3 Hz, 2H, Ar-H), 6.84 (d, J = 8.3 Hz, 2H, Ar-H), 6.75 (d, J = 8.8 Hz, 1H, Ar-H), 6.07 (s, 2H, NH2), 4.30 (s, 2H, SCH2), 3.82 (s, 3H, CH2-Ar-OCH3), 3.72 (s, 3H, NH-pyridine-OCH3), 2.50 (s, 2H, CH2CH3), 1.01 (t, J = 7.1 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.4, 159.9, 159.0, 158.1, 157.8, 140.1, 134.4, 131.3, 130.7, 130.2, 113.6, 109.3, 104.2 (C pyrimidine, pyridine and benzene), 55.0 (CH2-Ar-OCH3), 53.1 (NH-pyridine-OCH3), 31.9 (SCH2), 18.0 (CH2CH3), 12.8 (CH2CH3). HR-MS (ESI): Calcd. for C20H24N5O2S [M + H]+: 398.1651, found: 398.1651.
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-(3,4,5-trimethoxyphenyl)pyrimidine-2,4-diamine (XIIw) brown-red solid, yield 50.19%; mp 176.1–176.9°C; 1H NMR (400 MHz, DMSO-d6) δ 7.83 (s, 1H, NH), 7.34 (d, J = 8.2 Hz, 2H, Ar-H), 7.13 (s, 2H, Ar-H), 6.85 (d, J = 8.3 Hz, 2H, Ar-H), 6.09 (s, 2H, NH2), 4.31 (s, 2H, SCH2), 3.77 (s, 6H, 2OCH3), 3.72 (s, 3H, OCH3), 3.62 (s, 3H, OCH3), 2.53 (d, J = 7.8 Hz, 2H, CH2CH3), 1.00 (t, J = 7.1 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.5, 159.7, 158.1, 157.5, 152.2, 136.6, 132.5, 130.7, 130.2, 113.7, 104.7, 99.1 (C pyrimidine and benzene), 60.0 (OCH3), 55.7 (2OCH3), 55.0 (OCH3), 32.0 (SCH2), 17.9 (CH2CH3), 12.9 (CH2CH3). HR-MS (ESI): Calcd. for C23H29N4O4S [M + H]+: 457.1910, found: 457.1910.
5-Ethyl-6-((4-methoxybenzyl)thio)-N4-(4-nitrophenyl)pyrimidine-2,4-diamine (XIIx) yellow solid, yield 77.34%, mp 120.5–121.4°C; 1H NMR (400 MHz, DMSO-d6) δ 8.64 (s, 1H, NH), 8.13 (d, J = 9.3 Hz, 2H, Ar-H), 8.06 (d, J = 9.3 Hz, 2H, Ar-H), 7.35 (d, J = 8.3 Hz, 2H, Ar-H), 6.85 (d, J = 8.4 Hz, 2H, Ar-H), 6.40 (s, 2H, NH2), 4.33 (s, 2H, SCH2), 3.72 (s, 3H, OCH3), 2.58 (d, J = 7.5 Hz, 2H, CH2CH3), 1.00 (t, J = 7.2 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 167.1, 165.6, 159.8, 158.2, 156.5, 147.8, 140.4, 130.3, 124.3, 119.4, 113.7, 106.3 (C pyrimidine and benzene), 55.0 (OCH3), 32.3 (SCH2), 18.0 (CH2CH3), 13.0 (CH2CH3). HR-MS (ESI): Calcd. for C20H22N5O3S [M + H]+: 412.1443, found: 412.1443.
CONCLUSIONS
In summary, a series of 2-amino-5-ethylpyrimidine derivatives were designed and synthesized. Their antiproliferative activities against four human cancer cells were evaluated by MTT assay. Among the synthesized compounds, compound (XIIw) showed optimal antiproliferative activity against MGC-803 cells (IC50 = 1.02 ± 0.16 μM), and weaker cytotoxicity to GES-1 compared with the positive control 5-Fluorouracil (5-Fu). Meanwhile, compound (XIIw) could inhibit colony formation. Moreover, cell cycle experiments showed that compound (XIIw) blocked the cell cycle in S phase. Finally, DAPI staining and apoptosis experiments indicated that compound (XIIw) induced apoptosis of MGC-803 cells. All the evidences suggested that compound (XIIw) could be a potential antitumor agent.
REFERENCES
Cortes, J., Perez-García, J.M., Llombart-Cussac, A., Curigliano, G., El Saghir, N.S., Cardoso, F., Barrios, C.H., Wagle, S., Roman, J., Harbeck, N., Eniu, A., Kaufman, P.A., Tabernero, J., García-Estévez, L., Schmid, P., and Arribas, J., CA: Cancer J. Clin., 2020, vol. 70, pp. 105–124. https://doi.org/10.3322/caac.21597
Hernández-López, A., Téllez-González, M.A., Mondragón-Terán, P., and Meneses-Acosta, A., Front. Pharmacol., 2021, vol. 12, 720692. https://doi.org/10.3389/fphar.2021.720692
Arruebo, M., Vilaboa, N., Saez-Gutierrez, B., Lambea, J., Tres, A., Valladares, M., and Gonzalez-Fernandez, A., Cancers (Basel), 2011, vol. 3, pp. 3279–3330. https://doi.org/10.3390/cancers3033279
Fu, Z., Wang, L., Li, S., Chen, F., Au-Yeung, K.K.-W., and Shi, C., Front. Pharmacol., 2021, vol. 12, 736323. https://doi.org/10.3389/fphar.2021.736323
Sung, H., Ferlay, J., Siegel, R.L., Laversanne, M., Soerjomataram, I., Jemal, A., and Bray, F., CA: Cancer J. Clin, 2021, vol. 71, pp. 209–249. https://doi.org/10.3322/caac.21660
Kang, D., Zhang, H., Wang, Z., Zhao, T., Ginex, T., Luque, F.J., Yang, Y., Wu, G., Feng, D., Wei, F., Zhang, J., De Clercq, E., Pannecouque, C., Chen, C.H., Lee, K.H., Murugan, N.A., Steitz, T.A., Zhan, P., and Liu, X., J. Med. Chem., 2019, vol. 62, pp. 1484–1501. https://doi.org/10.1021/acs.jmedchem.8b01656
Regenass, P., Abboud, D., Daubeuf, F., Lehalle, C., Gizzi, P., Riche, S., Hachet-Haas, M., Rohmer, F., Gasparik, V., Boeglin, D., Haiech, J., Knehans, T., Rognan, D., Heissler, D., Marsol, C., Villa, P., Galzi, J.L., Hibert, M., Frossard, N., and Bonnet, D., J. Med. Chem., 2018, vol. 61, pp. 7671–7686. https://doi.org/10.1021/acs.jmedchem.8b00657
Ryan, S.J. and Yang, Q., Org. Process Res. Dev., 2019, vol. 23, pp. 2157–2165. https://doi.org/10.1021/acs.oprd.9b00130
Ward, R.A., Anderton, M.J., Bethel, P., Breed, J., Cook, C., Davies, E.J., Dobson, A., Dong, Z., Fairley, G., Farrington, P., Feron, L., Flemington, V., Gibbons, F.D., Graham, M.A., Greenwood, R., Hanson, L., Hopcroft, P., Howells, R., Hudson, J., James, M., Jones, C.D., Jones, C.R., Li, Y., Lamont, S., Lewis, R., Lindsay, N., McCabe, J., McGuire, T., Rawlins, P., Roberts, K., Sandin, L., Simpson, I., Swallow, S., Tang, J., Tomkinson, G., Tonge, M., Wang, Z., and Zhai, B., J. Med. Chem., 2019, vol. 62, pp. 11004–11018. https://doi.org/10.1021/acs.jmedchem.9b01295
Zhang, Y., Zhang, L., Liu, L., Wang, T., Meng, Y., Li, N., Li, E., Wang, Z., Liu, X., Zheng, J., Shan, L., Liu, H., and Zhang, Q., Chin. J. Org. Chem., 2020, vol. 40, pp. 1731–1736. https://doi.org/10.6023/cjoc202001034
Bedi, S., Khan, S.A., AbuKhader, M.M., Alam, P., Siddiqui, N.A., and Husain, A., Saudi Pharm. J., 2018, vol. 26, pp. 755–763. https://doi.org/10.1016/j.jsps.2018.04.010
Chan, C.M., Jing, X., Pike, L.A., Zhou, Q., Lim, D.J., Sams, S.B., Lund, G.S., Sharma, V., Haugen, B.R., and Schweppe, R.E., Clin. Cancer Res., 2012, vol. 18, pp. 3580–3591. https://doi.org/10.1158/1078-0432.CCR-11-3359
Garcia-Ferrer, M., Wojnicz, A., Mejia, G., Koller, D., Zubiaur, P., and Abad-Santos, F., Clin. Ther., 2019, vol. 41, pp. 2558–2570. https://doi.org/10.1016/j.clinthera.2019.10.009
Kumar, M., Kulshrestha, R., Singh, N., and Jaggi, A.S., Pharmacol. Res., 2019, vol. 143, pp. 86–96. https://doi.org/10.1016/j.phrs.2019.03.014
Zhang, S., Anjum, R., Squillace, R., Nadworny, S., Zhou, T., Keats, J., Ning, Y., Wardwell, S.D., Miller, D., Song, Y., Eichinger, L., Moran, L., Huang, W.S., Liu, S., Zou, D., Wang, Y., Mohemmad, Q., Jang, H.G., Ye, E., Narasimhan, N., Wang, F., Miret, J., Zhu, X., Clackson, T., Dalgarno, D., Shakespeare, W.C., and Rivera, V.M., Clin. Cancer Res., 2016, vol. 22, pp. 5527–5538. https://doi.org/10.1158/1078-0432.CCR-16-0569
Di Lello, P., Pastor, R., Murray, J.M., Blake, R.A., Cohen, F., Crawford, T.D., Drobnick, J., Drummond, J., Kategaya, L., Kleinheinz, T., Maurer, T., Rouge, L., Zhao, X., Wertz, I., Ndubaku, C., and Tsui, V., J. Med. Chem., 2017, vol. 60, pp. 10056–10070. https://doi.org/10.1021/acs.jmedchem.7b01293
Kategaya, L., Di Lello, P., Rouge, L., Pastor, R., Clark, K.R., Drummond, J., Kleinheinz, T., Lin, E., Upton, J.P., Prakash, S., Heideker, J., McCleland, M., Ritorto, M.S., Alessi, D.R., Trost, M., Bainbridge, T.W., Kwok, M.C.M., Ma, T.P., Stiffler, Z., Brasher, B., Tang, Y., Jaishankar, P., Hearn, B.R., Renslo, A.R., Arkin, M.R., Cohen, F., Yu, K., Peale, F., Gnad, F., Chang, M.T., Klijn, C., Blackwood, E., Martin, S.E., Forrest, W.F., Ernst, J.A., Ndubaku, C., Wang, X., Beresini, M.H., Tsui, V., Schwerdtfeger, C., Blake, R.A., Murray, J., Maurer, T., and Wertz, I.E., Nature, 2017, vol. 550, pp. 534–538. https://doi.org/10.1038/nature24006
Eldehna, W.M., Abou-Seri, S.M., El Kerdawy, A.M., Ayyad, R.R., Hamdy, A.M., Ghabbour, H.A., Ali, M.M., and Abou El Ella, D.A., Eur. J. Med. Chem., 2016, vol. 113, pp. 50–62. https://doi.org/10.1016/j.ejmech.2016.02.029
Fu, D.J., Cui, X.X., Zhu, T., Zhang, Y.B., Hu, Y.Y., Zhang, L.R., Wang, S.H. and Zhang, S.Y., Bioorg. Chem., 2021, vol. 107, p. 104634. https://doi.org/10.1016/j.bioorg.2021.104634
Zhang, Q., Teng, Y., Yuan, Y., Ruan, T., Wang, Q., Gao, X., Zhou, Y., Han, K., Yu, P., and Lu, K., Eur. J. Med. Chem., 2018, vol. 156, pp. 800–814. https://doi.org/10.1016/j.ejmech.2018.07.032
Yang, L., Liang, Q., Shen, K., Ma, L., An, N., Deng, W., Fei, Z., and Liu, J., Biomed. Pharmacother., 2015, vol. 71, pp. 70–78. https://doi.org/10.1016/j.biopha.2015.02.019
Jansa, P., Holy, A., Dracinsky, M., Kolman, V., Janeba, Z., Kostecka, P., Kmonickova, E., and Zidek, Z., Med. Chem. Res., 2014, vol. 23, pp. 4482–4490. https://doi.org/10.1007/s00044-014-1018-9
Legraverend, M., Ngongo-Tekam, R.M., Bisagni, E. and Zerial, A., J. Med. Chem., 1985, vol. 28, pp. 1477–1480. https://doi.org/10.1021/jm00148a017
Legraverend, M. and Bisagni, E., Tetrahedron Lett., 1985, vol. 26, pp. 2001–2002. https://doi.org/10.1016/s0040-4039(00)98364-3
Funding
Project supported by the National Natural Science Foundation of China (no. 81773562) and this work was supported by National Key Research Program of Proteins (no. 2018YFE0195100) and Openning fund from State Key Laboratory of Esophageal Cancer Prevention and Treatment (No. K2020000X).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The work has no studies involving humans or animals as subjects of the study.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Supplementary Information
Rights and permissions
About this article
Cite this article
Chao Gao, Dai, H., Si, X. et al. Synthesis and Antitumor Activity Evaluation of Novel 2-Amino-5-Ethylpyrimidine Derivatives. Russ J Bioorg Chem 48, 411–422 (2022). https://doi.org/10.1134/S1068162022020066
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022020066