Abstract
Two ZnO thin films have been chemically elaborated on glass substrates by successive ionic layer adsorption and reaction method using two different complexing agents, ammonia and ammonium hydroxide. X-ray diffraction study confirmed the hexagonal wurtzite structure for both films that are polycrystalline with preferential direction (002). Scanning electron microscopy showed an agglomeration of small grains throughout the substrate surfaces, with morphological changes and the existence of an uncovered part of substrates. The film prepared using NH4OH showed a higher transmittance. The optical band gap values for the films are close to 3.36 and 3.33 eV, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
The zinc oxide is a very important semiconductor, n-type, with a direct band gap close to 3.4 eV, and it crystallizes in the hexagonal wurtzite structure (a = 0.325 nm, c = 0.521 nm). ZnO also has attracted extensive attention because of its interesting properties, such as electrical and optical properties and high stability.
Due to its various and excellent properties, ZnO thin film is used in several devices such as UV light-emitting diodes, gas sensor electronics, and thin film solar cells. In addition, ZnO thin film is characterized by high transmittance in the visible region and low resistivity and is suitable to be used in solar cells as a good optical window. It can be obtained by many deposition techniques such as sputtering [1], pulsed laser deposition [2], sol–gel method [3], [4], chemical bath deposition (CBD) [5–7], spin coating [8, 9], and spray pyrolysis [10], and successive ionic layer adsorption and reaction (SILAR) [11, 12] have been utilized to get ZnO films. The SILAR technique presented by Nicolau [13] is a single technique, by which thin films of compound semiconductors can be synthesized off and on by means of the immersing substrate into the aqueous solutions containing ions for each component. But in our case, we used only two beakers, the first one contained a precursor solution based on the complexation of ions, and the second one contained hot distilled water for rinsing. The motivation behind this present study is to investigate the effect of complexing agents on the chemically synthesized ZnO thin films properties, such as structural, morphological, and optical properties by using two different complexing agents, namely ammonia NH3 and ammonium hydroxide NH4OH.
2 EXPERIMENTAL DETAILS
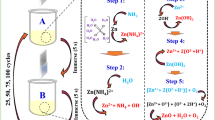
ZnO thin films were deposited on glass substrates (microscope slides) by chemical deposition process by alternate dipping into precursor solution kept at room temperature, by using 0.08 M of ZnSO4, and into hot distilled water maintained at 85°C. The precursor solutions were prepared by using zinc sulfate ZnSO4 and addition of 6 mL/100 mL 13.15 M ammonia or ammonium hydroxide. Firstly, the required amount of ZnSO4 was dissolved in distilled water in a glass beaker. Complexing agent (NH3 or NH4OH) was then added into this solution under constant stirring. At first, a white precipitate appeared in the solution due to the formation of zinc hydroxide Zn(OH)2, which dissolved on further addition of complexing agent. Prior to use, the glass substrates 75 × 25 × 1.5 mm3 were cleaned with acetone followed by distilled water. The following reactions occur in the two cases leading to the formation of ZnO:
(i) Using ammonia NH3: By immersing the substrate in the ZnSO4 and ammonia solution, the first beaker, we obtained the zinc ammonia complex ([Zn(NH3)4]2+) that is formed by Zn2+ and ammonia. In the first beaker, zinc ammonia was formed and adsorbed onto substrate. Then, the substrate was soaked in hot distilled water, second beaker, for rinsing the ions excess pre-adsorbed with formation of zinc hydroxide Zn(OH)2, which is gotten by the conversion of zinc ammonia complex [14]. The period of each immersion, in the first and the second beaker, is 30 s. Using the SILAR method, the detailed chemical reactions for the formation of ZnO are as follows:
So as to consider the transformation of Zn(OH)2 during the annealing, it could be written in the Kofstadt notation [15–17]:
The Zn(OH)2 will transform to ZnO by annealing in atmosphere:
(ii) Using ammonium hydroxide NH4OH: We keep the same parameters, replacing only NH3 with NH4OH, which leads to the formation of the hydroxide phase as follows:
To avoid precipitation and make the solution clear and transparent, the ammonium hydroxide is added to reduce Zn2+ and produce the complex ions Zn(NH3)\(_{n}^{{2 + }}\) (n = 1, …, 4) and n = 4 the most stable coordination number [18, 19]. This can be clarified as follows [20]:
Due to an attractive force between the ions in the solution and the glass substrate surface, these complex zinc ions are adsorbed on the substrate. These forces are van der Waals or chemical attractive or cohesive forces [21, 22]. Afterwards, the substrate is immersed in the second beaker containing hot distilled water for rinsing the excess of pre-adsorbed ions. This is accompanied by decomposition of the complex ion [Zn(NH3)4]2+ and formation of Zn(OH)2:
What’s more, inevitably, the formation of ZnO by transformation of the as-deposited Zn(OH)2 follows:
For both depositions, 20 deposition cycles and 30-s immersion period are required. The film prepared using ammonia NH3 has been referred to as Film no. 1, and that prepared using ammonium hydroxide NH4OH has been referred to as Film no. 2 in the text.
After deposition, the films were dried in air and annealed at 400°C for 2 h to remove the hydroxide phase included, if any. The annealed films were then subjected to various characterizations. The structural analysis of the ZnO thin films was carried out by the X-ray diffraction (XRD). The XPERT-PRO PW-3064 X-ray diffractometer using CuKα radiation with wavelength λ = 1.540598 Å was used for recording the XRD patterns. The optical measurements were accomplished by using the Perkin Elmer Instrument Lambda 900 UV/VIS/NIR spectrophotometer.
3 RESULTS AND DISCUSSION
3.1 Structural Studies
Figure 1 represents the XRD spectra of the chemically deposited ZnO thin films using complexing agents NH3 and NH4OH, which shows that the films are polycrystalline possessing hexagonal wiirtzite structure (ASTM card [01-079-0208, a = 3.2648 Å, and c = 5.2194 Å]).
The XRD patterns of both samples are dominated by a strong (002) diffraction peak, which suggests that the ZnO structure is preferably oriented along the c axis [23–25].
No phase corresponding to any other element or compound is observed in the XRD spectra. In addition to (002) plane, for Film no. 1, there are five other diffraction peaks corresponding to (100), (101), (102), (110), and (103). For Film no. 2, in addition to preferred orientation (002), presented are two other diffraction peaks only, (100) and (101). Comparing the diffraction peaks of both previous samples, the decrease of two diffraction peaks (100) and (101) is observed, and the increase of (002) diffraction peak, which means that the second sample (Film no. 2) has better crystalline quality.
3.2 Morphological Studies
Figure 2 shows the scanning electron micrographs of two ZnO thin films deposited on glass substrates using the complexing agents NH3 and NH4OH.
The SEM image of the Film no. 1, using NH3 as a complexing agent, exhibits small grains throughout the surface, which take different shapes such as small flowers and nanoprisms.
These grains are composed of small crystallites of hexagonal wurtzite structure, which is confirmed by previous XRD spectra. And it is observed that this film is relatively not dense with the existence of glass substrate surfaces that are not fully covered by the ZnO particles despite their large quantities. The SEM image of the Film no. 2, using NH4OH as a complexing agent, shows apparent agglomeration of small grains throughout the surface. And generally, it can be seen from the SEM images that this sample is denser than the first one.
3.3 Optical Studies
Figure 3 shows the absorbance and transmittance spectra of the ZnO thin films using different complexing agents.
Figure 3a reveals a low absorbance in the visible for both ZnO thin films. Usually, the optical absorption at the absorption edge corresponds to the transmission from the valence band to the conduction band, while the absorption in the visible region corresponds to some localized energy states in the band gap [26]. Figure 3b reveals the transmittance spectra in the visible region for the same thin films. According to this figure, it can be observed that the prepared ZnO Film no. 2, using NH4OH as a complexing agent, has a higher transmittance than the first one no. 1 (using NH3 as a complexing agent), and reaches 84%. As it follows from the analysis of the XRD spectra (Fig. 1b), this sample has the higher orientation along the c-axis, which will lead to improved optical characteristics. Transmittance is also affected by the number and size of surface formations and precipitates [24].
The theory of optical absorption gives the relationship between the absorption coefficient α and the incident photon energy hν for direct allowed transition as [21, 27, 28]:
with absorption coefficient α, photon energy E = hν, optical band gap Eg, and a proportionality constant A. Band gap values were obtained by extrapolating linear segments of α2 curves up to the intersection with hν axis.
The corresponding normalized plots are shown in Fig. 4 together with extrapolative straight line segments, producing two different values of optical band gap, i.e., 3.36 and 3.33 eV, for prepared ZnO thin films nos. 1 and 2, respectively, that are comparable to that of wurtzite bulk ZnO (3.37 eV).
The calculated band gap values well agree with other reports [29].
In general, the use of complexing agent NH4OH for elaborating ZnO thin films promotes the crystallization in comparison with the use of complexing agent NH3.
4 CONCLUSIONS
ZnO thin films grown on glass substrates by the SILAR method using two different complexing agents, namely, ammonia and ammonium hydroxide, are found to have hexagonal wurtzite structure with (002) preferred orientation along the c-axis. The surface morphology studies revealed that Film no. 1 has lower density with the presence of empty, i.e., the substrate surface was not well covered in comparison with Film no. 2. Optical studies showed low absorbance and high transmittance in the visible region, with band gap values of 3.36 and 3.33 eV for films no. 1 and no. 2, respectively.
REFERENCES
S. Kim, M.-C. Jeong, B.-Y. Oh, W. Lee, and J.-M. Myoung, J. Cryst. Growth 290, 485 (2006).
B. Q. Cao, M. Lorenz, A. Rahm, H. von Wenckstern, C. Czekalla, J. Lenzner, G. Benndorf, and M. Grundmann, Nanotechnology 18, 455707 (2007).
J. Zhao, Z. G. Jin, T. Li, and X. X. Liu, Appl. Surf. Sci. 252, 8287 (2006).
A. Jain, P. Sagar, and R. M. Mehra, Mater. Sci.-Pol. 25, 233 (2007).
C. D. Lokhande, P. M. Gondkar, R. S. Mane, V. R. Shinde, and S.-H. Han, J. Alloys Compd. 475, 304 (2009).
Q. F. Zhang, C. S. Dandeneau, X. Y. Zhou, and G. Z. Cao, Adv. Mater. 21, 4087 (2009).
A. Yamada, H. Miyazaki, Y. Chiba, and M. Konagai, Thin Solid Films 480–481, 503 (2005).
P. Popielarski, L. Mosinska, W. Bala, K. Paprocki, Y. Zorenko, T. Zorenko, and M. Sypniewska, Opt. Mater. 97, 109343 (2019).
S. Denchitcharoen, N. Siriphongsapak, and P. Limsuwan, Mater. Today Proc. 4, 6146 (2017).
R. Grothe, S. Knust, D. Meinderink, M. Voigt, A. G. Orive, and G. Grundmeier, Surf. Coat. Technol. 394, 125869 (2020).
V. L. Patil, S. A. Vanalakar, P. S. Patil, and J. H. Kim, Sens. Actuators, B 239, 1185 (2017).
S. S. Zahirullah, P. Immanuel, S. Pravinraj, P. F. H. Inbaraj, and J. J. Prince, Mater. Lett. 230, 1 (2018).
Y. F. Nicolau, Appl. Surf. Sci. 22–23, 1061 (1985).
A. Raidou, F. Benmalek, T. Sall, M. Aggour, A. Qachaou, L. Laanab, and M. Fahoume, Open Access Libr. J. 1, e588 (2014).
A. Jiménez-González and R. Suárez-Parra, J. Cryst. Growth 167, 649 (1996).
P. Kofstadt, Nonstoichiometry, Diffusion and Electrical Conductivity in Binary Metal Oxides (Wiley-Interscience, New York, 1972).
F. H. Kroeger and H. Vink, Solid State Physics (Academic, New York, 1956).
L. G. Sillen and A. E. Martell, Stability Constants of Metal-Ion Complexes: Supplement, Special Publication (Chem. Soc., London, 1971).
M. Qaiser Fatmi, T. S. Hofer, and B. M. Rode, Phys. Chem. Chem. Phys. 12, 9713 (2010).
M. A. Gaikwad, M. P. Suryawanshi, S. S. Nikam, C. H. Bhosale, J. H. Kim, and A. V. Moholkar, J. Photochem. Photobiol. Chem. 329, 246 (2016).
A. Raidou, F. Benmalek, T. Sall, M. Aggour, A. Qachaou, L. Laanab, and M. Fahoume, Opt. Quantum Electron. 46, 171 (2014).
H. M. Pathan and C. D. Lokhande, Bull. Mater. Sci. 27, 85 (2004).
M. A. Gaikwad, M. P. Suryawanshi, P. S. Maldar, T. D. Dongale, and A. V. Moholkar, Opt. Mater. 78, 325 (2018).
M. R. Cruz, R. Alfaro Garza-Hernández, P. P. Horley, J. Mata-Rami[acute]rez, E. Martínez, and F. S. Aguirre-Tostado, Thin Solid Films 663, 49 (2018).
S. Fairose and S. Ernest, Phys. B (Amsterdam, Neth.) 557, 63 (2019).
C. S. Prajapati and P. P. Sahay, Appl. Surf. Sci. 258, 2823 (2012).
S. Rezabeigy, M. Behboudnia, and N. Nobari, Proc. Mater. Sci. 11, 364 (2015).
J. Tauc, Mater. Res. Bull. 3, 37 (1968).
S. K. Shaikh, V. V. Ganbavle, S. V. Mohite, and K. Y. Rajpure, Thin Solid Films 642, 232 (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Raidou, A. Effect of Complexing Agents on Structural, Morphological, and Optical Properties of Chemically Deposited ZnO Thin Films. Semiconductors 55, 691–695 (2021). https://doi.org/10.1134/S1063782621080157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063782621080157