Abstract
A surface structural analysis of two ceramic samples of zirconium oxide (ZrO2) sintered at 1550°C, stabilized in the tetragonal form, before and after hydrothermal treatment, has been performed by the Rietveld method based on X-ray diffraction (XRD) data. The first sample was zirconium oxide with addition of ytterbium oxide (3 mol %), and the second sample contained ytterbium (3 mol %) and neodymium (0.25 mol %) oxides. The hydrothermal treatment is found to change the phase composition. In the initial state, there are two tetragonal ZrO2 forms (t and t'). The processes of surface-layer dissolution and crystallization of the monoclinic ZrO2 modification occur during hydrothermal treatment. ZrO2 crystals are shaped as plates elongated in the [111] direction, with developed {001} faces. Addition of neodymium oxide slows down the monoclinic-phase formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Ceramics based on solid solutions of zirconium dioxide, stabilized in the tetragonal form, belong to the class of transformation toughened materials. Due to the polymorphic transformations in the monoclinic–tetragonal–cubic series of zirconium dioxide modifications, occurring with an increase in volume, these materials exhibit high resistance to brittle fracture. Their crack resistance coefficient reaches 20 MPa m1/2, which is much higher than the corresponding values for ceramics of other types [1]. Currently, ceramics based on a solid solution of tetragonal zirconium dioxide, stabilized with yttrium oxide, is used in endoprosthetics of joints [2, 3] and in dental orthopedics [4]. The strength characteristics of this ceramics under long-term in vivo conditions were found to be unstable because of the change in its phase composition. This effect was referred to as low-temperature degradation [5–7]. One can eliminate it by changing the type of stabilizing cation and modifying the ceramics. In particular, the possibility of replacing zirconium with ytterbium was demonstrated in [8, 9]. According to the X-ray diffraction (XRD) data, three tetragonal ZrO2 forms may arise at different substitutions: t, t', and t''; they are characterized by the same sp. gr. P42/nmc. These forms differ by the degree of distortion of the ideal cubic fluorite cell [10–12].
The purpose of this study was to determine the structure of the surface layer of ceramics based on a ZrO2 solid solution, stabilized in the tetragonal form by adding only ytterbium oxide (3 mol % Yb2O3) of a mixture of neodymium and ytterbium oxides (3 mol % Yb2O3 and 0.25 mol % Nd2O3), before and after hydrothermal treatment, which imitated long-term in vivo conditions.
EXPERIMENTAL
The starting powders of two compositions (97 mol % ZrO2, 3 mol % Yb2O3 and 96.75 mol % ZrO2, 3 mol % Yb2O3, 0.25 mol % Nd2O3) were prepared by hydrolytic sol–gel synthesis. Hydrogels were precipitated simultaneously from a mixture of 1 M solutions of ZrOCl2, Yb(NO3)3, and Nd(NO3)3 salts using 6 N ammonia solution, and the obtained precipitates were filtered, washed from anion residues, and dried in air at a temperature of 180°C in ethanol environment. Agents of analytical grade were used. The heat treatment of xerogels was performed at a temperature of 950°C. Preforms for sintering were pressed into disks 18 mm in diameter using semi-dry method at a specific pressure of 200 MPa. The obtained preforms were sintered at a temperature of 1550°C for 4 h in electrical furnaces in air. After sintering the samples with a relative density of no less than 98% of the theoretical value were obtained (samples 1 and 2). They were subjected to hydrothermal treatment according to the accelerated aging technique (ISO 13356-2008) under the following conditions: temperature 134°C, pressure 2 bar, and exposure 5 h (samples 1h and 2h).
The diffraction patterns of all samples were recorded using an Ultma IV X-ray diffractometer (Rigaku, Japan) under the same conditions: voltage 40 kV; current 30 mA; CuKα radiation; nickel filter; D/tex high-speed detector; 2.5° Soller slits; 0.5° slit, which limits the incident-beam divergence; detector movement speed 2 deg/min; and step 0.01°. XRD analysis was carried out according to the Rietveld method using the PowderCell [13] and FullProf [14] packages. The parameters in refinement were the zero point with respect to the scattering angle, background component (fifth-order polynomial), scaling factors, profile characteristics of diffraction peaks, unit-cell parameters, atomic coordinates, and isotropic thermal factors. Since insignificant amounts of additives were introduced, the calculations were performed on the assumption of complete occupation of the corresponding crystallographic sites with zirconium atoms.
The element composition of samples 1 and 2 was monitored using an Orbis X-ray spectroscopic microanalyzer (EDAX, United States), which yielded good coincidence between calculated and experimental values. The contents of metals were calculated to be 94.0 at % Zr and 6.0 at % Yb in sample 1 and 93.5 at % Zr, 6.0 at % Yb, and 0.5 at % Nd in sample 2. The corresponding values, determined in the absence of reference, were 94.0 at % Zr, 1.0 at % Hf, and 5.0 at % Yb for sample 1 and 93.0 at % Zr, 1.0 at % Hf, 5.5 at % Yb, and 0.5 at % Nd for sample 2. The microscopic analysis of the surface of samples 1h and 2h was performed in a MIRA3 scanning electron microscope (Tescan, the Czech Republic).
RESULTS AND DISCUSSION
The starting crystallographic data for tetragonal forms t and t' were taken from [11]. Initially, it was erroneously suggested that samples 1 and 2 contain only form t. The presence of two forms t and t' is clearly demonstrated in Fig. 1, which shows the corresponding diffraction peaks (002) and (110). After preliminary analysis using the PowderCell software, the final refinement was performed within the FullProf program. The graphical refinement results for samples 1 and 2 are shown in Fig. 2, and the main numerical results are presented in Table 1. One can see in Table 1 that the phase compositions of samples 1 and 2 are close: the content of form t is about 60 vol %; the rest is form t'. The distortion of the initial cubic fluorite cell, which is estimated as the c/аf ratio, in form t exceed that in form t'. The refined values of the atomic coordinates and thermal factors of tetragonal forms t and t', present in samples 1 and 2, are listed in Table 2. Zr and O atoms are characterized by much higher thermal factors in forms t' than in forms t, which is indicative of atomic disordering at occupation of the corresponding sites (presence of vacancies).
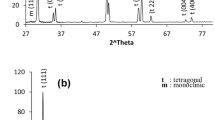
Hydrothermal treatment of the ceramic samples containing the tetragonal ZrO2 form induces occurrence of the monoclinic modification [8, 9, 15]. This phase was additionally introduced to the considered model before carrying out Rietveld refinement. The crystallographic data were taken from [16]. The refinement results for samples 1h and 2h are shown graphically in Fig. 3, and the main numerical results are presented in Table 1. The monoclinic ZrO2 (m) modification, which is present in samples 1h and 2h, has some specific features. A powder ZrO2 sample without addition of Yb2O3 (maximum firing temperature 950°C) and a ceramic sample of monoclinic ZrO2 with addition of 1 mol % Yb2O3 (sintering temperature 1550°C) were also prepared to determine these features. According to the data of [17], the maximum Yb2O3 solubility in ZrO2 is specifically 1 mol %. The unit-cell parameters of the monoclinic phases, which are present in additionally prepared samples 1h and 2h, are compared with the data from [16] in Table 3. One can see that the unit-cell volume and parameter a of the samples after hydrothermal treatment are larger than the corresponding parameters of the additional samples with and without 1% Yb2O3. These facts are in agreement with the data of [15] and the suggestion about incorporation of OH– hydroxyl groups into the crystal structure of monoclinic phases. The refined values of atomic coordinates and thermal factors of the monoclinic phases existing in samples 1h and 2h are listed in Table 4. One can see their good coincidence and good correspondence with the data of [16]. Another feature of these monoclinic phases is that the relative intensities of the diffraction peaks are inconsistent with the data from the PDF2 database (in particular, card 83-940). This inconsistency is clearly demonstrated in Fig. 4. The (\(\overline 1 \)11) peak is much stronger than the (111) peak of the monoclinic phase of sample 1h, while in the case of powder ZrO2 sample these peaks have close intensities. The same situation is also observed for the (011) and (110) peaks. These phenomena are due to the lamellar shape of the m-ZrO2 crystals formed during hydrothermal treatment. The plates are elongated in the [111] direction and have developed {001} faces. The corresponding corrections were introduced into the refined Rietveld model, which made it possible to reach low reliability factors (Table 1). The monoclinic phase of sample 2h also exhibits this specific feature. It was reported previously [18, 19] that the m-ZrO2 crystals formed as a result of the hydrothermal treatment are elongated in the [111] direction and have a lamellar or needle-like shape. It should also be noted that the diffraction peaks corresponding to the monoclinic phases of samples 1h and 2h are broadened in comparison with the peaks of tetragonal forms. This broadening may be due to the small size of the coherent-scattering region, which is usually related to the geometric size of particles. To verify this suggestion, calculations for the strongest (\(\bar {1}\)11) peak of the monoclinic phases of samples 1h and 2h were performed using the WinFit program [20]. A ceramic ZrO2 sample with addition of 1 mol % Yb2O3, sintered at 1550°C, was used as a reference sample. The coherent-scattering regions were 83 and 87 nm in size, respectively.
The results of scanning electron microscopy are presented in Fig. 5. The chosen portion is characteristic of samples 1h and 2h. It can be seen that the surface of sample 1h after hydrothermal treatment is irregular: it has ridges and valleys, formed as a result of partial dissolution of the surface layer. The ceramic grains are mainly about 0.5 µm in size; however, individual grains reach 3–5 µm. This grain size is characteristic of ZrO2 ceramics stabilized into the tetragonal form after sintering at 1500°C [21]. No ZrO2 crystals of monoclinic modification were observed at the chosen magnification.
CONCLUSIONS
The XRD analysis according to the Rietveld method showed that, after sintering at 1550°C, the ceramic samples, consisting of yttrium oxide with addition of ytterbium oxide (3 mol %) and oxides of ytterbium (3 mol %) and neodymium (0.25 mol %), contain two tetragonal ZrO2 forms (t and t '). The phase composition of the surface changes, and dissolution and crystallization processes of the monoclinic ZrO2 modification occur during hydrothermal treatment. Two tetragonal forms (t and t ') and the monoclinic phase (m) are present on the sample surface. The monoclinic crystals are shaped as plates elongated in the [111] direction, with the developed {001} faces. Addition of neodymium oxide slows down the monoclinic-phase formation.
REFERENCES
J. Chevalier, A. Liens, H. Reveron, et al., J. Am. Ceram. Soc. 103, 1482 (2020). https://doi.org/10.1111/jace.16903
C. Piconi and G. Maccauro, Biomaterials 20, 1 (1999). https://doi.org/10.1016/S0142-961(98)00010-6
J. Chevalier, Biomaterials 27, 535 (2006). https://doi.org/10.1016/j.biomaterials.2005.07.034
J. R. Kelly and I. Denry, Dental Mater. 24, 289 (2008). https://doi.org/10.1016/j.dental.2007.05.005
L. Borshers, M. Stiesch, F.-W. Bach, et al., Acta Biomater. 6, 4547 (2010). https://doi.org/10.1016/j.actbio.2010.07.025
M. Cattani-Lorente, S. Scherrer, P. Ammann, et al., Acta Biomater. 7, 858 (2011). https://doi.org/10.1016/j.actbio.2010.09.020
P. Kohorst, L. Borchers, J. Strempel, et al., Acta Biomater. 8, 1213 (2012). https://doi.org/10.1016/j.actbio.2011.11.016
L. I. Podzorova, S. A. Titov, A. A. Il’icheva, et al., Materialovedenie, No. 7, 52 (2015).
L. I. Podzorova, A. A. Il’icheva, N. A. Mikhailinoi, et al., Perspekt. Mater., No. 2, 27 (2017).
M. Yashima, N. Ishizawa, and M. Yoshimura, J. Am. Ceram. Soc. 76, 641 (1993). https://doi.org/10.1111/j.1151-2916.1993.tb03654.x
M. Yashima, S. Sasaki, M. Kakihana, et al., Acta Crystallogr. B 50, 663 (1994). https://doi.org/10.1107/S0108768194006257
M. Yashima, M. Kakihana, and M. Yoshimura, Solid State Ionics 86–88, 1131 (1996). https://doi.org/10.1016/0167-2738(96)00386-4
W. Kraus and G. Nolze, J. Appl. Crystallogr. 29, 301 (1996). https://doi.org/10.1107/S0021889895014920
J. Rodriguez-Carvajal, Physica B 192, 55 (1993). https://doi.org/10.1016/0921-4526(93)90108-1
M. Yoshimura, T. Noma, K. Kawabata, and S. Somiya, J. Mater. Sci. Lett. 6, 465 (1987). https://doi.org/10.1007/BF01756800
M. Winterer, R. Delaplane, and R. McGreevy, J. Appl. Crystallogr. 35, 434 (2002). https://doi.org/10.1107/S0021889802006829
M. Gonzalez, C. Moure, J. R. Jurado, and P. Duran, J. Mater. Sci. 28, 3451 (1993). https://doi.org/10.1007/BF01159821
L. Kumari and W. Z. Li, Cryst. Growth Des. 9, 3874 (2009). https://doi.org/10.1021/cg800711m
H. Nishizawa, N. Yamasaki, K. Matsuoka, and H. Mitsushio, J. Am. Ceram. Soc. 65, 343 (1982). https://doi.org/10.1111/j.1151-2916.1982.tb10467.x
S. Krumm, Mater. Sci. Forum. 228–231, 183 (1996). https://doi.org/10.4028 www.scientific.net/MSF.228-231
L. Hallmann, P. Ulmer, E. Reusser, et al., J. Eur. Ceram. Soc. 32, 4091 (2012). https://doi.org/10.1016/j.jeurceramsoc.2012.07.032
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by Yu. Sin’kov
Rights and permissions
About this article
Cite this article
Sirotinkin, V.P., Podzorova, L.I., Mikhailina, N.A. et al. X-ray Diffraction Study of Structural Changes in High-Strength Ceramics Based on Zirconium Oxide with Additions of Ytterbium and Neodymium Oxides after Hydrothermal Treatment. Crystallogr. Rep. 67, 278–285 (2022). https://doi.org/10.1134/S1063774522020171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774522020171