Abstract
The characteristics of the gas phase and the kinetics of reactive-ion etching of silicon in a 50% C6F12O + 50% Ar plasma are studied. The study scheme includes plasma diagnostics using Langmuir probes and optical emission spectroscopy, as well as the measurement of etching rates with varying input power (200–600 W) and gas pressure (4–12 mTorr). It is shown that (a) the nature of the change in the parameters of the electron and ion components of the plasma generally corresponds to the regularities known for other fluorocarbon gases; and (b) the kinetics of the formation of fluorine atoms is significantly affected by bulk processes of the form CFx + O → COFx–1 + F. It is established that the change in the silicon etching rate is determined by the kinetics of the heterogeneous reaction Si + xF → SiFx flowing in the mode of limitation by the flow of fluorine atoms. It is assumed that the effective probability of this reaction under constant temperature conditions is determined by the processes of competitive adsorption of oxygen atoms and/or surface oxidation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Nonequilibrium gas-discharge plasma of fluorocarbon (CxHyFz) gases historically plays an important role in the technology of integrated micro- and nanoelectronics devices during the processes of structuring the surface of various kinds of silicon wafers and functional layers [1, 2]. The main tool here consists of the processes of reactive ion etching (RIE), which combine the effects of physical and chemical effects on the treated surface. The control of physical and chemical factors under varying treatment conditions (the composition of the plasma gas, its pressure, input power, and displacement power on the treated surface) allow us to effectively optimize the etching rate, profile anisotropy, and selectivity with respect to the mask material [3, 4].
Obviously, optimization of the RIE output characteristics is impossible without understanding the mechanisms of the physical and chemical phenomena that ensure the gasification of the treated surface atoms. This, in turn, requires the identification of relationships between the parameters of the gas phase (electrophysical characteristics and plasma composition) and the kinetics of ion-stimulated heterogeneous processes. Numerous studies of relevant issues for the plasma of traditional fluorocarbon gases—CF4, CHF3, C4F8, etc.—are set out in the reviews [5–8] and summarized in the monographs [1–4]. In general, for each of these gases (a) kinetic schemes have been developed that adequately describe the kinetics of plasma-chemical processes and the composition of the gas phase; (b) the types of dominant particles are established and their roles in the etching process are determined; (c) the factors that determine the polymerization load of the plasma on the surfaces in contact with it, as well as the composition and stationary thickness of the fluorocarbon polymer film, are analyzed; and (d) methods have been developed to control the etch/polymerization balance by varying the external parameters and additive gases.
Recent studies have shown that a significant drawback of traditional fluorocarbon gases is their high global warming potential (GWP) [9, 10]. For example, the value of the GWP index (a parameter showing how many times the contribution of the given gas to the formation of the greenhouse effect exceeds the similar effect of CO2) is more than 5000 for tetrafluoromethane and more than 7000 for trifluoromethane [10, 11]. Thus, the search for new plasma-forming gases, which, on the one hand, have a lower negative impact on the environment, and, on the other hand, meet the requirements of the technology, is an urgent task. According to the results of the works [11, 12], we can conclude that one of the possible candidates here could be C6F12O, which has a unit GWP index and remains liquid at temperatures below 50°C. The latter property greatly facilitates the extraction of the unreacted fluorocarbon component from the exhaust gases of plasma-chemical reactors. At the same time, the properties C6F12O plasma and the possibilities of its technological applications have been studied extremely poorly. The main reasons for this are the lack of experimental data on the kinetics of RIE processes in the given gas, as well as the objective difficulties in analyzing the electrophysical parameters and plasma composition against the background of an indefinite kinetic scheme. The development of the latter is significantly hampered by the lack of reliable information on the mechanisms and kinetic characteristics (cross sections, rate constants) of the dissociation of C6F12O and its decay products.
Earlier in [13] a comparative study of the plasma parameters and kinetics of RIE of Si and SiO2 in CF4 + Ar and C6F12O + Ar mixtures was carried out. It was found that under identical discharge excitation conditions, both plasma systems (a) are characterized by the similar concentrations of charged particles; and (b) there are no fundamental differences in the composition or thickness of the deposited fluorocarbon polymer film. However, a distinctive feature of C6F12O + Ar plasma is the lower etching rates of Si and SiO2, which is consistent with the differences in the concentrations of fluorine atoms. At the same time, an obvious shortcoming of this work is the lack of data on the absolute concentrations of atoms. This limits the possibilities of analyzing the kinetics of neutral particles in the plasma volume and heterogeneous processes on the treated surface. The main idea of this study was to further develop the experimental and theoretical approaches to study the properties of C6F12O + Ar plasma under conditions typical for RIE processes of silicon and its compounds. Accordingly, the objectives of the study were (1) to obtain data on the absolute concentrations of fluorine and oxygen atoms with varying input power and gas pressure; and (2) analyze the silicon etching mechanism in the effective interaction probability approximation. As shown earlier in our works [14–16], the nature of the change in this parameter under the conditions of temperature control of the processed material makes it possible to identify third-party factors that affect the kinetics of a heterogeneous chemical reaction.
2 METHODOLOGICAL PART
2.1 Equipment and Experimental Technique
The experiments were carried out under the conditions of an induction RF (13.56 MHz) discharge in a planar reactor with an anodized aluminum working chamber [14–16]. The variable values were the gas pressure (\(p\) = 4–12 mTorr) and input power (\(W\) = 200–600 W), which corresponded to the specific power range of ~0.2 to 0.6 W/cm3. The set of constant parameters included the total flow rate of the plasma gas (\(q\) = 40 std. cm3/min), and the initial composition of the mixture C6F12O + Ar given by equal partial flow rates of its components, and the bias power at the lower electrode (\({{W}_{{dc}}}\) = 200 W). The last condition corresponded to the variable value of the bias voltage \( - {{U}_{{dc}}}\), which tracks the change in the positive ion flux density.
Data on the electrophysical parameters of the plasma were obtained using a double Langmuir probe DLP2000 (Plasmart Inc., Korea). To minimize the distortion of the probe current-voltage characteristics (CVC) due to polymerization on the probes, a pulsed ion bombardment cleaning system was used. In addition, before each measurement, the probes were additionally processed in 50% Ar + 50% O2 plasma for ∼2 minutes. An analysis of the measured CVCs using the well-known provisions of the probe theory for low-pressure discharges [4, 17] provided data on the electron temperature (\({{T}_{e}}\)) and ion current density (\({{J}_{ + }}\)). The total concentration of positive ions was estimated from the ratio \({{n}_{ + }} \approx {{J}_{ + }}{\text{/}}0.61e{{v}_{B}}\) [4], where \({{v}_{B}} \approx \sqrt {e{{T}_{e}}{\text{/}}{{m}_{i}}} \) is the ion velocity at the outer boundary of the electrical double layer near the probe surface and \({{m}_{i}}\) is the effective ion mass [15, 16]. When determining \({{m}_{i}}\) we assumed that the dominant ion in plasma C6F12O is \({\text{CF}}_{3}^{ + }\). Indirect confirmation of this fact is (a) the absence of fundamental differences in the absolute values \({{J}_{ + }}\), measured in C6F12O and CF4 under identical discharge excitation conditions [13]; and (b) CF4 dominance among stable thermal decomposition products of C6F12O, including under the conditions of the spark breakdown of the gas gap [18, 19]. The value \( - {{U}_{{dc}}}\) was controlled with an AMN-CTR high-voltage probe (Youngsin Eng, Korea).
Stationary concentrations of F and O atoms were determined by optical actinometry using analytical pairs F 703.8 nm/Ar 750.4 nm and O 777.2 nm/Ar 750.4 nm [20, 21]. All these spectral lines are characterized by (a) excitation by direct electron impact; and (b) a low lifetime of the excited state, which makes it possible to neglect the processes of nonradiative relaxation [21]. The atomic concentrations were calculated from the relation
where \({{I}_{X}}\) and \({{n}_{X}}\) are the radiation intensity and concentration of the determined particle X (X = F or O), and \(C_{{Ar}}^{X}\) is the actinometric coefficient determined by the ratio of the excitation constants and the probabilities of optical transitions. The excitation cross sections and the parameters of optical transitions are well known from the literature. [21, 22]. In particular, in the work [21], it was shown that parameters \(C_{{Ar}}^{X}\) are constant in the electron temperature range of 3 to 4 eV (∼2 for a pair of F 703.8 nm/Ar 750.4 nm and ∼1.6 for the O 777.2 nm/Ar 750.4 nm pair), while the results of the actinometric determination of atomic concentrations are in close agreement with the results of mass spectral measurements.
The samples subjected to etching were located in the central part of the lower electrode and consisted of fragments of Si(100) wafers of ∼2 × 2 cm. The built-in water cooling system maintained a constant electrode temperature after plasma ignition. The etching rate was determined as \(R = h{\text{/}}\tau \), where \(\Delta h\) was the height of the step at the border of the masked and nonmasked areas of the treated surface, and \(\tau ~\) was the etching time. Value \(\Delta h\) was measured with an Alpha-step D-500 profilometer (KLA-Tencor, United States), and an AZ1512 photoresist ∼1.5 µm thick was used as a masking coating. The value of the etching time was chosen within the quasi-linear section of the kinetic dependence \(\Delta h = f\left( \tau \right)\) corresponding to the stationary etching mode. Obviously, the existence of such a mode is ensured by the constancy of the sample temperature and the absence of the effect of masking the treated surface with a fluorocarbon polymer. According to the results of preliminary experiments, it was also found that an increase in the treated surface area does not lead to a decrease in the etching rate and is not accompanied by noticeable changes in the probe CVC. This allows us to conclude that the etching process proceeds in the kinetic mode and is characterized by a negligibly small effect of the etching products on the parameters of the gas phase.
2.2 Approaches to the Analysis of Etching Kinetics
The analysis of the relationship between the parameters of the gas phase and the kinetics of heterogeneous interaction was based on the published data on the mechanisms of reactive-ion processes in the plasma of fluorocarbon gases [4–7, 14, 23]. For typical RIE conditions (gas pressure <20 mTorr, ion bombardment energy 200–600 eV), these data can be summarized in the following statements:
(1) The observed RIE rate can be represented as \({{R}_{{phys}}} + {{R}_{{chem}}}\), where the terms are the rates of physical sputtering and chemical etching of the target material.
(2) The physical component of the etching process (\({{R}_{{phys}}}\) and any ion-stimulated process, for example, desorption of the interaction products or destruction of a fluorocarbon polymer film) is characterized by the rate \({{Y}_{S}}{{{{\Gamma }}}_{ + }}\), where \({{Y}_{S}}\) is the output of the process (atom/ion) and \({{{{\Gamma }}}_{ + }} \approx {{J}_{ + }}{\text{/}}e\) is the ion flux density. The change in parameter \({{Y}_{S}}\) when varying the RIE conditions is proportional to \(\sqrt {{{\varepsilon }_{i}}} \), where \({{\varepsilon }_{i}} = e\left| { - {{U}_{f}} - {{U}_{{dc}}}} \right|\) is the bombarding ion energy and \({{U}_{f}}\) is the floating potential.
(3) The chemical component of the etching process (\({{R}_{{chem}}}\) and any heterogeneous reaction involving neutral active particles, for example, the formation of a fluorocarbon polymer film or its etching with oxygen atoms) is characterized by the rate \({{\gamma }_{X}}{{{{\Gamma }}}_{X}}\), where \({{\gamma }_{X}}\) is the effective interaction probability and \({{{{\Gamma }}}_{X}}\) is the flux density of the corresponding neutral particles. The change in the parameter \({{\gamma }_{X}}\) for the target material under varying RIE conditions is determined by the kinetics of the processes of filling and cleaning active centers. The key factors here are the surface temperature, the volatility of the interaction products, and the presence of processes leading to the inhibition or masking of active centers. The competitive adsorption of nonreacting particles is usually considered as the first mechanism and the deposition of a fluorocarbon polymer film is considered as the second one.
3 RESULTS AND DISCUSSION
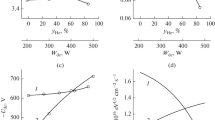
According to the results of probe diagnostics of C6F12O + Ar plasma, it was found that the nature of the change in the parameters of the electron and ion components of the plasma corresponded to the regularities known for other fluorocarbon gases. From Table 1, it can be seen that the electron temperature and ion current density increase with increasing power added at \(p\) = const, but decrease with increasing gas pressure under the conditions \(W\) = const. The nature of dependence \({{T}_{e}}~ = ~f\left( W \right)\) is due to the increase in the degrees of dissociation of the molecular components of the plasma, which leads to the enrichment of the gas phase with atomic particles and less saturated CFx radicals. Therefore, there is a decrease in the energy losses of electrons in low-threshold processes of vibrational and electronic excitation. Accordingly, the drop in \({{T}_{e}}\) with increasing gas pressure reflects the opposite change in the energy loss of electrons due to the increase in the frequency of their collisions with heavy particles. It can also be seen that the dependences \({{J}_{ + }} = f\left( {p,~W} \right)\) are always sharper than the value \(\sqrt {{{T}_{e}}} \), which tracks the behavior of the ion velocity at the boundary of the electrical double layer near the probe surface. Thus, the observed responses of the ion current density to a change in the discharge excitation conditions are generally formed by similar dependences of the total concentration of positive ions \({{n}_{ + }}\) and hence the ionization rate. The growth of the invested power at \(p\) = const increases the total ionization rate due to a similar change in the electron concentration in the plasma. The unambiguous dependency \({{n}_{e}} = f\left( W \right)\) follows from the balance equation for the input power, which is analyzed in [4, 24]. In contrast, the increase in gas pressure under the conditions \(W\) = const suppresses ionization by reducing \({{T}_{e}}\) and the rate constants of the corresponding processes \({{k}_{{iz}}}\). The characteristic values of the threshold energies of processes of the form CFx + e → \({\text{CF}}_{x}^{ + }\) + 2e (especially for the ionization of atomic particles) exceed the average energy of electrons in plasma \(\varepsilon \) ≈ 3/2\({{T}_{e}}\). Therefore, even small changes in the electron temperature are accompanied by sharp dependences \({{k}_{{iz}}} = f\left( {{{T}_{e}}} \right)\). Another factor that reduces the efficiency of the formation of positive ions with increasing gas pressure can be the drop in the electron density due to an increase in the electronegativity of the plasma. The reason for this may be related, among other things, to an increase in the concentrations of the F2 and O2 molecules formed during the recombination of fluorine and oxygen atoms. Note also that the magnitude of the negative bias under the conditions \({{W}_{{dc}}}\) = const always changes inversely \({{J}_{ + }}\). This is due to the partial compensation of the induced negative charge due to the heterogeneous electron-ion recombination.
When studying the emission spectra of the plasma, it was found that the emission intensities of atomic lines F 703.8 nm, O 777.2 nm, and Ar 750.4 nm sharply increase with increasing input power, but slightly decrease with increasing gas pressure (Fig. 1a). Obviously, both effects are a superposition of changes in the concentrations of these particles and excitation functions \({{k}_{{ex}}}{{n}_{e}}\), where \({{k}_{{ex}}} = f\left( {{{T}_{e}}} \right)\) is the excitation rate constant. The actual concentrations of F and O atoms determined from the results of actinometric measurements (Fig. 1b), have a less pronounced dependence on the value W, but increase with increasing gas pressure. The reason for the opposite effect of pressure on the intensity of radiation and the concentration of atoms is the sharper decrease in the corresponding excitation functions.
The analysis of data Fig. 1b suggests some features of the kinetics of fluorine and oxygen atoms in C6F12O + Ar plasma. First, the effect of the input power on the concentration of fluorine atoms is always much stronger than for oxygen atoms. At low pressures, this is expressed in different trends in the growth of the concentrations of the corresponding particles (by factors of ∼2.5 for \({{n}_{F}}\) and ∼1.5 for \({{n}_{O}}\) at \(p\) = 4 mTorr and \(W\) = 200–600 W); and at high pressures, in an even sharper increase of \({{n}_{F}}\) at \({{n}_{O}}\) ≈ const. In our opinion, this means that a significant contribution to the formation of fluorine atoms is made by bulk processes of the R1 form: CFx + O → COFx–1 + F (\({{k}_{1}}\) ∼ 3 × 10–11 cm3/s for \(x\) = 3, 2 and ∼ 6 × 10–11 cm3/s for \(x\) = 1 [25, 26]). Accordingly, the transition to the region of high pressures increases the rate of oxygen consumption due to the increase in the concentrations of CFx radicals. Second, note that in the region \(W\) < 450–500 W, the condition \({{n}_{O}}\) > \({{n}_{F}}\) is always satisfied. It is logical to assume that the dissociation of the initial molecule C6F12O predominantly comes with a break in the weakest C–C bonds (∼610 kJ/mol or 6.4 eV) and С–F bonds (∼552 kJ/mol or 5.8 eV) [22], while the strong C=O double bond (∼1060 kJ/mol or 11 eV) ensures the formation of CO molecules as one of the stable dissociation products. Thus, the higher concentrations of oxygen atoms can be due to the binding of fluorine in bulk R2 processes: CO + F → CFO (\({{k}_{2}}\) ∼ 3 × 10–11 cm3/s) and R3: CFO + F → CF2O (\({{k}_{3}}\) ∼ 8 × 10–11 cm3/s) [26, 27]. Obviously, an increase in the input power reduces the efficiency of these channels due to the increase in the rates of CO dissociation by electron impact.
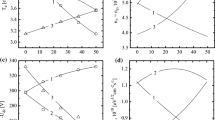
Figure 2 presents data illustrating the effect of discharge excitation conditions on the kinetics of silicon etching. The measured etch rates \({{R}_{{Si}}}\) increase monotonically both with an increase in the input power and gas pressure, which contradicts the nature of the change in the ion flux density (Table 2). Thus, in the studied range of conditions, the etching process does not have ion-limited stages and is determined by the kinetics of the heterogeneous reaction R4: Si + xF → SiFx. The latter conclusion is confirmed by estimates of sputtering rates \({{R}_{{phys}}}\) using published data on the dependence of the sputtering coefficient on the energy of ions [28] (Table 2). Calculations of the effective probability of heterogeneous interaction \(\gamma = {{R}_{{chem}}}{\text{/}}{{{{\Gamma }}}_{F}}\), where \({{R}_{{chem}}} = {{R}_{{Si}}} - {{R}_{{phys}}}\) is the chemical component of the etching rate and \({{{{\Gamma }}}_{F}} \approx 0.25{{n}_{F}}{{\left( {8R{{T}_{{gas}}}{\text{/}}\pi {{M}_{F}}} \right)}^{{1/2}}}\) is the flux density of fluorine atoms, showed the dependence of this parameter on the discharge excitation conditions even under conditions of the temperature control of the sample in the reactor (Fig. 2b). Previously, in [13], it was found that the plasma polymerization ability C6F12O + Ar has no fundamental differences from similar characteristics of the CF4 + Ar system. Since the properties of the latter are well known, it can be assumed with a sufficient degree of confidence that the condition \({{\varepsilon }_{i}}\) > 200 eV ensures the existence of a thin or even noncontinuous polymer film, which does not limit the access of fluorine atoms to the treated surface [4]. In our opinion, the reason for the decrease in parameter \(\gamma \) with an increase in the input power and gas pressure, is the competitive adsorption of oxygen atoms, leading to a decrease in the proportion of free active centers. In addition, it is logical to assume the occurrence of chemical processes involving oxygen, which reduce the reactivity of silicon with respect to fluorine atoms. As such processes, we can consider (a) oxidation of the surface itself, leading to the formation of Si–O bonds, which formally corresponds to an increase in the threshold energy R4; and (b) oxidation of R4 products into less volatile compounds of the SiFxOy type, which leads to a decrease in the proportion of active centers capable of adsorbing fluorine atoms. Note that similar heterogeneous effects were discussed earlier for mixtures of traditional fluorocarbon gases with oxygen. For example, in [29], a similar correlation was observed between the concentration of oxygen atoms and the probability of interaction during the etching of SiO2 in a CF4 + O2 + Ar plasma mixture by varying the O2/Ar ratio. A similar situation has also been reported for Si and SiO2 in a C4F8 + O2 + Ar mixture under conditions where the etching rate is not limited by the diffusion of fluorine atoms in the polymer layer [15, 30]. In the light of the foregoing, the slight increase in the effective probability of interaction in the low-pressure region at \(W\) = 200–400 W (Fig. 2b) is presumably the result of ionic activation of the process through the purification of active centers and the breaking of oxide bonds. In the region of high pressures, this is hindered by the combination of a low flux density of ions and a high flux density of oxygen atoms. For the same reason, there is a decrease in parameter \(\gamma \) with an increase in gas pressure under conditions \(W~\) = const.
Silicon etch rates (a) and the effective probability of the heterogeneous reaction Si + xF → SiFx (b) in a plasma mixture of 50% C6F12O + 50% Ar. (a) (1, 2) Measured etching rates \(R\); (3, 4) chemical component of the etching rate \({{R}_{{chem}}}\) defined as \({{R}_{{Si}}} - {{R}_{{phys}}}\). Solid lines correspond to a gas pressure of 4 mTorr; dotted lines correspond to 12 mTorr.
In conclusion, we note that the conclusions made in this work on the mechanisms of the gas-phase and heterogeneous processes in C6F12O + Ar plasma are rather hypothetical due to the lack of direct experimental evidence. At the same time, they generally agree with the results of studies of other fluorocarbon gases and, apparently, adequately reflect the specifics of the system under study.
4 CONCLUSIONS
A study was made of the electrophysical parameters of the plasma, the concentrations of atomic components, and the kinetics of reactive-ion etching of silicon in a mixture of C6F12O + Ar. Interest in C6F12O is due to the prospect of replacing traditional fluorocarbon gases, which have high global warming potentials. In an experimental study of the gas phase using Langmuir probes and optical emission spectroscopy, it was found that (a) the nature of the change in the parameters of the electronic and ion components of the plasma corresponds to the patterns known for other fluorocarbon gases; (b) the concentration of fluorine atoms increases monotonically with an increase in the input power and gas pressure; and (c) volumetric processes of the CFx + O → COFx–1 + F form affect the kinetics of the formation of fluorine atoms. It is shown that the chemical component makes a dominant contribution to the silicon etching process, while the kinetics of the heterogeneous Si + xF → SiFx reaction are characterized by a nonconstant effective interaction probability. It is assumed that the decrease in the effective probability with increasing gas pressure is due to the inhibitory effect of oxygen atoms through the processes of competitive adsorption and/or surface oxidation.
REFERENCES
Roosmalen, J., Baggerman, J.A.G., and Brader, H.S.J., Dry Etching for VLSI, New York: Plenum, 1991.
Wolf, S. and Tauber, R.N., Silicon Processing for the VLSI Era, Vol. 1: Process Technology, New York: Lattice Press, 2000.
Nojiri, K., Dry Etching Technology for Semiconductors, Tokyo: Springer, 2015.
Lieberman, M.A. and Lichtenberg, A.J., Principles of Plasma Discharges and Materials Processing, New York: Wiley, 1994.
Jansen, H., Gardeniers, H., de Boer, M., Elwenspoek, M., and Fluitman, J., A survey on the reactive ion etching of silicon in microtechnology, J. Micromech. Microeng., 1996, vol. 6, pp. 14–28.
Stoffels, W.W., Stoffels, E., and Tachibana, K., Polymerization of fluorocarbons in reactive ion etching plasmas, J. Vac. Sci. Technol. A, 1998, vol. 16, pp. 87–95.
Kay, E., Coburn, J., Dilks, A., Plasma chemistry of fluorocarbons as related to plasma etching and plasma polymerization, in Plasma Chemistry III, Veprek, S. and Venugopalan, M., Eds., Vol. 94 of Topics in Current Chemistry, Berlin: Springer, 1980.
Donnelly, V.M. and Kornblit, A., Plasma etching: Yesterday, today, and tomorrow, J. Vac. Sci. Technol., 2013, vol. 31, p. 050825–48.
Muhle, J., Ganesan, A.L., Miller, B.R., et al., Perfluorocarbons in the global atmosphere: Tetrafluoromethane, hexafluoroethane, and octafluoropropane, Atmos. Chem. Phys., 2010, vol. 10, pp. 5145–5164.
Tran-Quinn, T. and Lakritz, M., Unsaturated fluorocarbons in the etching process, environmental benefit, technical hurdles, in Proceedings of 2008 IEEE/SEMI Advanced Semiconductor Manufacturing Conference, Cambridge, USA, May 5–7, 2008, pp. 37–42.
Mocella, M.T., PFC emission control options for plasma processing tools: A current assessment, MRS Online Proc. Library, 1996, vol. 447, pp. 29–34.
Krishnan, N., Smati, R., Raoux, S., and Dornfeld, D., Alternatives to reduce perfluorinated compound (PFC) emissions from semiconductor dielectric etch processes: Meeting environmental commitments while minimizing costs, in Proceedings of IEEE International Symposium on Electronics and the Environment, Boston, USA, May 19–22, 2003, pp. 19–24.
Lim, N., Cho, Y.S., Efremov, A., and Kwon, K.-H., Dry etching performance and gas-phase parameters of C6F12O + Ar plasma in comparison with CF4 + Ar, Materials, 2021, vol. 14, p. 1595-1–16.
Efremov, A., Murin, D., and Kwon, K.-H., Concerning the effect of type of fluorocarbon gas on the output characteristics of the reactive-ion etching process, Russ. Microelectron., 2020, vol. 49, no. 3, pp. 157–165.
Lee, B.J., Efremov, A., Nam, Y., and Kwon, K.-H., Plasma parameters and silicon etching kinetics in C4F8 + O2 + Ar gas mixture: Effect of component mixing ratios, Plasma Chem. Plasma Process., 2020, vol. 40, pp. 1365–1380.
Efremov, A., Murin, D., and Kwon, K.-H., Plasma parameters, densities of active species and etching kinetics in C4F8 + Ar gas mixture, Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol., 2019, vol. 62, no. 2, pp. 31–37.
Shun’ko, E.V., Langmuir Probe in Theory and Practice, Boca Raton: Universal Publ., 2008.
Li, Y., Zhang, X., Tian, S., Xiao, S., Li, Y., and Chen, D., Insight into the decomposition mechanism of C6F12O–CO2 gas mixture, Chem. Eng. J., 2019, vol. 360, pp. 929–940.
Zhang, X., Tian, S., Xiao, S., Deng, Z., Li, Y., and Tang, J., Insulation strength and decomposition characteristics of a C6F12O and N2 gas mixture, Energies, 2017, vol. 10, p. 1170-1–11.
Booth, J.P. and Sadeghi, N., Oxygen and fluorine atom kinetics in electron cyclotron resonance plasmas by time-resolved actinometry, J. Appl. Phys., 1991, vol. 70, pp. 611–620.
Lopaev, D.V., Volynets, A.V., Zyryanov, S.M., Zotovich, A.I., and Rakhimov, A.T., Actinometry of O, N and F atoms, J. Phys. D: Appl. Phys., 2017, vol. 50, p. 075202-1–17.
Handbook of Chemistry and Physics, Boca Raton, FL: CRC, 1998.
Gray, D.C., Tepermeister, I., and Sawin, H.H., Phenomenological modeling of ion-enhanced surface kinetics in fluorine-based plasma-etching, J. Vac. Sci. Technol. B, 1993, vol. 11, pp. 1243–1257.
Efremov, A.M., Kim, G.H., Kim, J.G., Bogomolov, A.V., and Kim, C.I., Applicability of self-consistent global model for characterization of inductively coupled Cl2 plasma, Vaccum, 2007, vol. 81, no. 5, pp. 669–675.
Kimura, T. and Noto, M., Experimental study and global model of inductively coupled CF4/O2 discharges, J. Appl. Phys., 2006, vol. 100, pp. 063303-1–9.
Efremov, A., Lee, J., and Kim, J., On the control of plasma parameters and active species kinetics in CF4 + O2 + Ar gas mixture by CF4/O2 and O2/Ar mixing ratios, Plasma Chem. Plasma Process., 2017, vol. 37, pp. 1445–1462.
Chun, I., Efremov, A., Yeom, G.Y., and Kwon, K.-H., A comparative study of CF4/O2/Ar and C4F8/O2/Ar plasmas for dry etching applications, Thin Solid Films, 2015, vol. 579, pp. 136–143.
A Simple Sputter Yield Calculator. http://www.iap. tuwien.ac.at/www/surface/sputteryield. Accessed January 20, 2022.
Son, J., Efremov, A., Chun, I., Yeom, G.Y., and Kwon, K.-H., On the LPCVD-formed SiO2 etching mechanism in CF4/Ar/O2 inductively coupled plasmas: Effects of gas mixing ratios and gas pressure, Plasma Chem. Plasma Process., 2014, vol. 34, pp. 239–257.
Lee, B.J., Efremov, A., and Kwon, K.-H., Gas-phase chemistry and reactive-ion etching kinetics for silicon-based materials in C4F8 + O2 + Ar plasma, Plasma Process. Polym., 2021, vol. 18, no. 7, p. e2000249-1–17.
Funding
This study is part of a state task of the Federal State Institution Scientific Research Institute for System Analysis, Russian Academy of Sciences (conducting fundamental scientific research (47 GP)) on topic no. 11021060909091-4-1.2.1 “Fundamental and applied research in the field of lithographic limits of semiconductor technologies and physical and chemical etching processes of 3D nanometer dielectric structures for the development of critical production technologies of the ECB. Research and construction of models and designs of microelectronic elements in an extended temperature range (from –60°C to +300°C) (FNEF-2022-0006).”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Efremov, A.M., Betelin, V.B. & Kwon, KH. Plasma Parameters and Kinetics of Reactive-Ion Etching of Silicon in a C6F12O + Ar Mixture. Russ Microelectron 51, 247–254 (2022). https://doi.org/10.1134/S1063739722040047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063739722040047