Abstract
The dependence among the content of energy reserves in Sorex caecutiens, the abundance of food objects, and the number of shrews in the summer periods of 2012–2019 was estimated. The glycogen content in the liver of S. caecutiens did not show a significant relationship to the number of animals. The mass of fat depots and the relative biomass of invertebrates (according to the counting data obtained with the pitfall traps) in August were inversely related to the total number of all species of shrews inhabiting the territory. The mass of the adipose tissue of S. caecutiens positively correlated with the biomass of insect larvae (primarily of caterpillar-like ones, which constituted the bulk of larvae in the samples). The revealed features indicate an increase in intra- and interspecific food competition during the years of high numbers of shrews.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCION
The abundance of shrews is subject to significant seasonal fluctuations. It is minimal in spring and grows rapidly in summer during the reproductive period. Upon reaching a peak in July–September, sometimes even before the end of the breeding season, numbers begin to decline. This decline lasts until the next summer, but its rate is not the same in different seasons of the year. The first significant decrease in the abundance of animals usually occurs in fall (Kalinin et al., 2008; Shchipanov et al., 2019). Many regions are also characterized by substantial interannual variations in the abundance of shrews. Often, density-dependent factors play an important role in the formation of their population dynamics, when the intensity of summer growth and/or the magnitude of the subsequent decline in numbers depend on the population density (Kaikusalo and Hanski, 1985; Henttonen et al., 1989; Sheftel, 1989; Dokuchaev, 1990; Huitu et al., 2004; Kalinin et al., 2008; Kiselev and Yamborko, 2014; Kiselev, 2019; Bobretsov et al., 2020). In the years with an increased number of animals, the seasonal peak and the beginning of a subsequent decrease occur at earlier dates (Kalinin et al., 2008). A high population density clearly has a negative impact on the shrew. It was noted that their overpopulation leads to an increase in the fluctuating asymmetry of the skeletal characteristics of individuals, which, according to the authors of the work, may be a consequence of an increase in the level of social stress (Zakharov et al., 1991, 1997). It was also assumed that at least one of the components of the density-dependent regulation of the shrew abundance may be the intensity of exploitation of food resources (Kaikusalo and Hanski, 1985; Moraleva, 1987; Henttonen et al., 1989; Kiselev et al., 2014; Kiselev and Yamborko, 2014).
The feeding conditions can affect both the survival rate and the intensity of reproduction in animal populations. This influence can be not only direct, but also indirect: through changes in the behavior of individuals, the spatial structure of populations, susceptibility to predators and pathogens, etc. (Shilov, 1998). The increase in mortality in response to poor feeding conditions in shrews can occur rather quickly. Shrews are small insectivorous mammals with an extremely high metabolic rate. The high level of metabolism, relatively small fat reserves, and the absence of such an energy-saving mechanism as torpor force these animals to feed frequently (Genoud, 1988; Taylor, 1998). Per day, shrews consume an amount of food that is almost equal to or even exceeds their own body mass (depending on the species), and their lifetime without access to food is only a few hours. This puts them in constant dependence on the availability of food items.
Theoretically, not only intra-, but also interspecific competition for food resources can affect the well-being of populations of shrews. They feed on a variety of invertebrates, the occurrence of which in their stomachs, as a rule, corresponds to their availability in nature. At the same time, the same territory is usually inhabited by several species of shrews, the diets of which almost completely overlap, and the differences in the composition of the prey they consume are not qualitative, but quantitative (Pernetta, 1976; Korolkova, 1977; Dokuchaev, 1981, 1990; Churchfield, 1982; Churchfield et al., 1991, 1999; Churchfield and Sheftel, 1994; Klenovšek et al., 2013; Dokuchaev et al., 2015; Ivanter et al., 2015). The differences in the body size of coexisting species contribute to a partial decrease in the interspecific competition in shrew communities (Kirkland, 1991; Fox and Kirkland, 1992). The body size affects the foraging mode (epigeal/hypogeal), as well as the type and size of prey consumed: the larger the shrew, the higher the frequency of eating larger invertebrates (Churchfield and Sheftel, 1994; Churchfield et al., 1999). Earthworms (Lumbricidae) play an important role in the diet of large shrew species.
Field experiments have shown that small mammals, especially shrews, can significantly affect certain food items. Fencing the experimental plots to exclude this animals significantly increased the abundance of some invertebrates in comparison with unfenced control plots (Korolkova, 1975, 1977; Churchfield et al., 1991; Namba and Ohdachi, 2016). The proportion of invertebrates taken by shrews, however, varies depending on the population density of the latter, the number of prey, the season of the year, and some other factors. In England, for example, no critical impact of shrews on invertebrates was noted; the total abundance of prey throughout the year remained at a fairly high level (Churchfield, 1982; Churchfield et al., 1991). It has been assumed that shrews never lack food (Pernetta, 1976; Churchfield, 1982). Moving northward, as the productivity of habitats decreases, the impact of shrews on forage sites increases (Korolkova, 1975). In the northern taiga, the significant role of insectivores in the decline of many groups of invertebrates was noted already in the summer period (Korolkova, 1975, 1977). In Karelia, the estimated share of forage removal by shrews at the peak of their abundance exceeded 50% (Ivanter and Makarov, 1994).
Despite the fact that in certain cases shrews can have a very significant effect on food objects, it is not known how this affects the animals themselves. Earlier, in the upper reaches of the Kolyma River (continental part of Northeast Asia), a dependence of the physiological state of Sorex caecutiens and Sorex isodon on the level of their numbers was noted (Kiselev et al., 2014). The relationship was found between the abundance of shrews and their energy reserves, indicating inadequate feeding of animals in the years of increased numbers. Invertebrates, however, have not been counted, and the exact cause of the observed deterioration in nutrition remains unclear. Besides the depletion of food resources, it was assumed that social interactions (aggressive contacts) between individuals and competition for space could hinder normal feeding.
The purpose of this study was (1) on the basis of an assessment of the physiological parameters (relative mass of interscapular and inguinal adipose tissue, glycogen content in the liver) to establish whether signs of inadequate feeding of S. caecutiens appear during the years of high numbers of shrews on the northern coast of the Sea of Okhotsk; (2) if so, to try to identify whether this is a consequence of an increase in the intrinsic population density or the abundance of all shrew species in aggregate; and (3) verify how the interannual variability of the physiological parameters of S. caecutiens correlates with the abundance of food items. S. caecutiens is the most abundant and widespread species among shrews in Northeast Asia; it reaches the highest abundance in larch forests (Dokuchaev, 1990). In addition to S. caecutiens, five species of shrews inhabit the northern coast of the Sea of Okhotsk: Sorex isodon, Sorex gracillimus, Sorex daphaenodon, Sorex camtschaticus, and Sorex minutissimus. The first of these species is the most numerous; the rest are minor. Fluctuations in abundance over the years in all species during the study period coincided to varying degrees (Kiselev, 2019).
MATERIALS AND METHODS
Study area and capture of shrews. This work was carried out on the northern coast of the Sea of Okhotsk in the vicinity of Magadan (59°37′ N, 150°56′ E). The climate of the study area is characterized by excessive moisture, cold summers, and snowy frosty winters (Klyukin, 1970). A more detailed description of the weather and climatic conditions was given earlier (Kiselev, 2019). This study was conducted on material collected in July–August 2012–2019 in the larch forest habitat preferred by S. caecutiens. The undergrowth in this forest is formed by Siberian Dwarf Pine and Middendorf birch, the ground cover is formed by mosses, shrubs and small shrubs (blueberries, ledum, lingonberries, etc.), and short grass. The animals were caught using cone pitfall traps half filled with water. The relative abundance estimation were carried out on a constant catch line (Kiselev, 2019), while additional traps (cones and beakers with a volume of 0.5 L) were also used to provide a larger amount of material according to the physiological parameters of shrews. The traps were checked every morning, the animals were immediately delivered to the laboratory, dried on napkins, and weighed on a balance with an accuracy of 0.01 g, after which the physiological parameters of shrews were analyzed.
Assessment of physiological parameters. Immature juveniles (the young of the year) of S. caecutiens were used in the analyses. Animals with signs of molting were excluded from the analysis, as this process affects the physiological parameters of shrews (Dokuchaev, 1983, 1990). Due to the fact that there are no significant sex differences in the studied parameters in young immature individuals of S. caecutiens (Kiselev et al., 2014), the data on males and females were analyzed together. A total of 320 specimens were included in the analysis.
The inguinal (on one side) and interscapular adipose tissues were weighed on a balance with an accuracy of 1 mg. The relative tissue mass was expressed in mg/g body. Glycogen was precipitated with ethanol after treatment of the liver with 30% KOH and hydrolyzed to glucose in 2 N H2SO4 (Davidson and Berliner, 1974). The amount of glucose in the hydrolyzate was determined by the orthotoluidine method. The glycogen content in the liver was expressed in mg of glucose per 1 g of fresh organ weight.
Estimation of the relative biomass of invertebrates. Invertebrates were caught in July–August using pitfall traps (Barber traps) located near traps for small mammals (in the same place in all years). Ten plastic beakers with a volume of 0.5 L and an inlet diameter of 90 mm were placed in a line (just below the soil surface level) at a distance of 5 m from each other and half filled with water. Collection of trapped invertebrates was carried out after seven days by passing the contents of the cups through a sieve. After that, the traps were re-installed in their original place until the next check. The collected invertebrates were divided into groups, dried on napkins until an approximately natural state, and weighed. The relative biomass of invertebrates was expressed in g/100 trap-nights. With the exception of larvae, invertebrates were divided into groups according to their taxonomic status. In connection with the sensitivity of the type of traps used to the mobility of objects, the larvae of insects (with complete metamorphosis) were divided into groups according to their appearance and ability to move. Worm-like (without developed limbs; mainly Diptera, Hymenoptera, and, to a lesser extent, Coleoptera), campodeiform (represented in the collections exclusively by Coleoptera), and caterpillar-like (Lepidoptera and sawfly) larvae were identified. Small and/or scarce invertebrates, which are almost always characterized by a low biomass in samples, were assigned to a separate group (other invertebrates).
Invertebrates that are not used or are hardly ever used for food by the shrews in Northern Priokhot’e (Dokuchaev, personal communication) were excluded from the analysis. These include adults and larvae of carrion beetles (Silphidae), red velvet mites (Trombidiidae), ants (Formicidae), and adults of large Coleoptera. The latter (with the exception of carrion beetles), however, were rare in the habitat studied; only once was a ground beetle of the genus Carabus with a body mass of 0.6 g caught. Data on actively flying invertebrates (adults of Diptera, Lepidoptera, Hymenoptera, etc.) were also not included in the analysis.
Statistical analysis. Statistical data processing was performed in Statistica 10.0 (StatSoft, Inc.). The assessment of the reliability of differences in body mass and adipose tissue between years and months was carried out using two-way ANOVA after log-transformation of the data. As the distribution of individuals by the content of glycogen in the liver was very different from the normal, and the transformation of the data did not correct the situation, the identification of interannual differences in this parameter was performed using the Kruskal‒Wallis test. Differences in the liver glycogen content between months were performed using the Mann‒Whitney test. The relationships between the relative abundance of shrews, their physiological parameters, and the relative abundance of invertebrates were assessed using Spearman’s rank correlation coefficient (Rs).
RESULTS
Population dynamics and variability of the physiological parameters of shrews. The relative abundance of shrews in the larch forest varied over the years within a fairly wide range (Fig. 1a). Fluctuations in the total abundance of all shrew species generally repeated those of S. caecutiens, which actually constituted the bulk of shrews in the habitat under consideration. Only in 2015, when the total abundance of shrews reached another peak, did the abundance of S. caecutiens, although it increased in relation to the previous year, still remain at a relatively low level. The highest abundance in the larch forest in 2015 was in S. isodon, which usually ranked second in abundance among shrews in this habitat (Kiselev, 2019).
Dynamics of the number of shrews and physiological parameters of S. caecutiens: (a) relative abundance (the solid line indicates S. caecutiens, the dashed line, all species of shrews in aggregate); (b) body mass; (c) relative mass of interscapular adipose tissue; (d) relative mass of the inguinal adipose tissue; and (e) content of glycogen in the liver. Light circles and columns are July, dark ones designate August. Data are presented as means and errors of means.
The physiological parameters of S. caecutiens are shown in Figs. 1b–1e. Two-way analysis of variance showed that the year, month, and their combined effect did not make a significant contribution to the variability of the body mass of the shrews. Other physiological parameters changed significantly over the years (interscapular adipose tissue: F7.304 = 4.7, p < 0.01; inguinal adipose tissue: F7.303 = 6.0, p < 0.01; liver glycogen: H7.266 = 47.7, p < 0.01). The influence of the month on the physiological parameters was not significant, but the combined effect of the month and the year had a significant effect on the relative mass of adipose tissue (interscapular adipose tissue: F7.304 = 2.9; p < 0.01; inguinal adipose tissue: F7.303 = 3.1; p < 0.01).
In both July and August, none of the physiological parameters in the interannual relation showed a significant relationship with the abundance of S. caecutiens. The relative mass of interscapular and inguinal adipose tissue negatively correlated with the total number of shrews, but only in August (Rs = –0.8, p < 0.05 and Rs = –0.9, p < 0.01, respectively). A positive relationship was established between the individual values of the mass of interscapular and inguinal adipose tissue (Rs = 0.8, p < 0.01 both in absolute value and relative to body mass). The glycogen content in the liver did not show significant correlations with the total number of shrews.
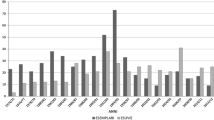
The dynamics of the relative biomass of invertebrates. The analysis of variability of food items biomass included nine groups of invertebrates: adults of beetles (Coleoptera), centipedes (Myriapoda), oligochaetes (Oligochaeta), spiders (Araneae), harvestmen (Opiliones), three groups of larvae, and the group of other invertebrates (Fig. 2). The highest relative biomass was observed in almost all years for harvestmen; only in 2016, with a surge in the number of Lepidoptera, were caterpillar-like larvae characterized by the highest biomass. Spiders in the habitat studied were few in July–August. Caterpillar-like larvae took the second place after harvestmen in biomass among invertebrates; the biomass of worm-like and campodeiform larvae in the samples was relatively low. The abundance of coleopterans (with the exception of carrion beetles that were not included in the analysis) in the studied habitat was also relatively low. Staphylinids (Staphylinidae) were the most abundant among coleopterans, but due to their small body size, their biomass was small. The myriapods (Myriapoda) took third place in terms of biomass after harvestmen and caterpillar-like larvae. In the overwhelming majority of the samples, they were represented by the order Lithobiomorpha (class Chilopoda); occasionally Geophilomorpha was encountered. Of the oligochaetes in the larch forest, only representatives of the family Enchytraeidae were found. Other invertebrates were mainly represented by springtails (Collembola), scale insects (Coccinea), aphids (Aphidinea), snow scorpionflies (Boreidae), and gastropods (Gastropoda). The relative biomass of this group usually did not reach 0.05 g/100 trap-nights, and only in 2019, when an outbreak of aphid abundance occurred, was this value significantly exceeded.
The dynamics of the relative biomass of individual groups of invertebrates and all invertebrates as a whole is shown, respectively, in Figs. 2 and 3. In July, no reliable relationships were found between the total biomass of food items or the biomass of individual groups of invertebrates and the abundance of S. caecutiens or the abundance of all shrew species together. In August, the total biomass of invertebrates showed an inverse dependence on the total abundance of shrews (Rs = ‒0.8; p < 0.05), but not on the abundance of S. caecutiens (Rs = –0.3; p > 0.05). Among individual groups of invertebrates, only the biomass of caterpillar-like larvae showed a significant negative relationship with both the total abundance of shrews and the abundance of S. caecutiens in August (Rs = –0.8; p < 0.05 in both cases). The biomass of all larvae in aggregate was also inversely related to the total abundance of shrews (Rs = –0.8; p < 0.05).
The physiological parameters of shrews did not show a significant relationship with the biomass of invertebrates in July. In August, a high positive correlation was found between the relative mass of adipose tissue of S. caecutiens and the biomass of caterpillar-like larvae (interscapular adipose tissue: Rs = 0.95; p < 0.01 and inguinal adipose tissue: Rs = 0.75; p < 0.05), as well as the biomass of all larvae in general (respectively, Rs = 0.95; p < 0.01 and Rs = 0.85; p < 0.01). In August, the physiological parameters did not show a significant relationship with the biomass of other groups of invertebrates or with the total biomass of food resources.
DISCUSSION
Dynamics of the relative biomass of invertebrates. The method of sampling invertebrates used is sensitive not only to their abundance, but also to their activity. All other things being equal, the catching efficiency for less active forms (for example, worm-like larvae) will be lower in comparison with invertebrates actively moving along the ground surface. Inactive forms of insects (pupae/cocoons, eggs) remained unaccounted for in this work. The traps used are also not suitable for sampling the inhabitants of deep soil layers (for example, earthworms and larvae of some Diptera). However, these invertebrates, although they constitute a significant part of the diet of large shrew species, are not very significant in the diet of small species, including S. caecutiens. The latter forage mainly in the ground surface/litter layer (Churchfield and Sheftel, 1994; Churchfield et al., 1999; Ivanter et al., 2015), and the abundance of invertebrates in traps should largely correspond to their availability for shrews. For example, in England, the composition and ratio of various groups of invertebrates in pitfall traps were similar to those in the diet of Eurasian pygmy shrews (Sorex minutus) (Pernetta, 1976), but to a much lesser extent corresponded to those in the diet of the common shrew (Sorex araneus) (Pernetta, 1976; Churchfield, 1982). For the second species, which is characterized by a relatively large body size, the composition and ratio of consumed prey were more consistent with those in the soil samples (Churchfield, 1982). In general, in the regions of permafrost soils of the extreme northeast of Asia, surface forms of invertebrates in terms of abundance significantly prevail over geobionts proper, but the latter are also concentrated in the upper soil horizon (Berman and Bukhkalo, 1985; Bukhkalo, 2009). In the sparse forest belt of the upper reaches of the Kolyma River, for example, below 10 cm of the layer in soil samples, only a few individuals of enchytraeids and larvae of flies were found occasionally (Berman and Bukhkalo, 1985).
The results of this study indicate that, even in the summer period, shrews can have a significant impact on the food resources, although the degree of this impact is not the same in different months, as well as in relation to different groups of invertebrates. The lack of reliable relationships between the relative indices of the number of shrews and the biomass of invertebrates in July may be due to the fact that at this time the abundance of shrews, as a rule, is still small. Accordingly, their impact on invertebrates may not be high enough to be reflected in the counts. In August, the relative biomass of all invertebrates in the aggregate did not show a relationship to the abundance of S. caecutiens, but was inversely related to the total abundance of different species of shrews. Among individual groups of invertebrates, only the biomass of caterpillar-like larvae showed a reliable dependence on the abundance of shrews. The latter prevailed significantly among the larvae in the samples. Their biomass on the ground surface, as a rule, decreased from July to August during the years with a high abundance of shrews, but increased in other years (Fig. 2). A rather high frequency of Lepidoptera caterpillars in the diet of shrews is typical for many regions (Churchfield and Sheftel, 1994; Volpert and Shadrina, 2002; Dokuchaev et al., 2015; Ivanter et al., 2015, etc.); this is the main food for Sorex cinereus (a species similar in size to S. caecutiens) in the pine forests of Eastern Canada (Bellocq et al., 1994; Bellocq and Smith, 2003). Sawfly larvae are also readily consumed by shrews (Korolkova, 1977; Dokuchaev, 1981, 1990; Ivanter et al., 2015). There is evidence that shrews can be very effective predators of lepidopterans and sawflies in the cocoon stage (Buckner, 1955, 1969; Holling, 1959; Korolkova, 1977; Hanski and Parviainen, 1985). In field experiments in Western Finland, the proportion of pine sawfly cocoons taken by small mammals (mainly the Laxmann’s shrew and the bank vole) positively correlated with the number of the latter in less productive forest types. In highly productive forest types, such a relationship was not manifested, despite the higher number of animals, probably due to the greater abundance of alternative food (Hanski and Parviainen, 1985).
The relative biomass of other groups of invertebrates separately, including the harvestmen, did not show a reliable relationship with the abundance of shrews. An analysis of the impact of shrews on the number of harvestmen was previously carried out in Karelia, where these invertebrates are also abundant and are actively consumed by insectivores (Makarov and Ivanter, 2016). According to the authors of this study, shrews begin to feed on harvestmen after the latter reach a certain size, while different species of shrews are included in the consumption of harvestmen at different times. As a result, the greatest pressure of predators on these arachnids falls on the period of the maximum number of prey, and the damage caused to them by shrews is not great. The abundance of harvestmen in Karelia showed an inverse dependence on the number of shrews only in comparison between different habitats. In the larch forest surveyed on the coast of the Sea of Okhotsk, there was a clear tendency toward a negative correlation between the abundance of shrews and the biomass of harvestmen in August. The exception was 2013, when, despite the high number of shrews, the biomass of harvestmen was also high. Thus, in the study area, shrews are likely to have a significant effect on the dynamics of the number of these invertebrates.
The adipose tissue of representatives of the genus Sorex is very peculiar. As shown for S. araneus and S. minutus, almost all fat deposits of shrews have a similar histological structure characteristic of brown fat (Hyvärinen, 1994). Brown adipose tissue differs structurally, metabolically, and functionally from white; its main function is the production of heat. At the same time, a number of indirect facts indicate that the adipose tissue of these insectivores also functions as white fat (Hyvärinen, 1994; Kiselev, 2017b). It was assumed (Kiselev, 2017b) that this tissue in shrews, at least in part, can be represented by the so-called beige or brite (brown in white) adipocytes. To clarify the features of the organization and functioning of the adipose tissue of shrews, special studies are necessary. In this case, it is important that the mass of fat reserves, at least in the warm season, can be a good indicator of the feeding conditions of this animals (Dokuchaev, 1990). The adipose tissue of shrews is very labile. Abundant feeding of captive animals leads to the rapid deposition of large amounts of fat reserves (Dokuchaev, 1990; Kiselev, unpublished data). Thus, already after two days of keeping in captivity (at room temperature), the mass of inguinal and interscapular adipose tissue in the S. isodon (n = 94), on average, exceeded that in animals in the natural environment by more than twofold, and in S. caecutiens (n = 12), by more than three times. Lack of access to food, on the other hand, leads to the rapid depletion of fat reserves. Eight-hour fasting in the experiment significantly reduced the mass of adipose tissue in S. isodon (Kiselev, 2017b). In the natural environment, the rate of exhaustion of fat reserves during fasting in shrews is even higher. As demonstrated for S. araneus, low environmental temperatures, high activity of individuals, and some other factors increase the metabolic rate of shrews; in natural conditions, it is close to the maximum possible for the species (Ochocińska and Taylor, 2005). In individuals that died of starvation in live traps and pitfall traps without water, as well as in snap traps, in the event of being hit on the tail or a paw, fat depots are practically absent (Dokuchaev, 1990). Due to low ambient temperatures and much lower initial energy reserves, the life span of shrews after the onset of fasting in this case is significantly lower than when kept in captivity (Dokuchaev, 1990; Shchipanov et al., 2019). A high rate of fat turnover in shrews was recently demonstrated experimentally based on the analysis of stable carbon isotopes in the breath of S. araneus (Keicher et al., 2017). All body fat of S. araneus in this study was turned over after 9–24 h, and the estimated time of fat storage depletion in the absence of access to food ranged from 4.2 to 11 h in different age groups. It is also obvious that different food items should have a different effect on the intensity of accumulation of fat reserves by these insectivores. Thus, the mass of adipose tissue can serve as a good indicator of the feeding conditions of shrews, although some other factors leading to an increase in energy expenditures can influence the fat reserves of this animals. For example, their decrease was noted during molting (Dokuchaev, 1990), with a decrease in ambient temperature (Kiselev, unpublished data).
In July, no significant relationship was found between the mass of adipose tissue and the number of shrews. In August, the mass of fat reserves of S. caecutiens did not show a significant relationship with its abundance, but it significantly (negatively) correlated with the total abundance of shrews, and also (positively) with the biomass of certain food items, namely, insect larvae (primarily caterpillar-like ones, which made up the bulk of the larvae in the samples). Thus, it can be concluded that the observed differences in the fatness of S. caecutiens in the years with different levels of shrew abundance are a manifestation of competitive relations. Insect larvae appear to have a high nutritional value. They are important preferred prey items for shrews (Korolkova, 1977; Churchfield, 1982; Churchfield and Sheftel, 1994; Volpert and Shadrina, 2002; Dokuchaev et al., 2015; Ivanter et al., 2015). The abundance of lepidopterans and sawfly larvae, however, could also correlate with the abundance of their pupae/cocoons, which could not be accounted for by the methods used for collecting the invertebrates. There are other data indicating the influence of the availability of insect larvae on the fat reserves of shrews. Dokuchaev (1990) noted a rapid decrease in the mass of adipose tissue of S. isodon in a poplar–chosenia forest after the emergence of adult crane flies (Tipulidae), the abundance of larvae and pupae of which was previously high.
The fact that the dependence of fat depot mass of S. caecutiens on the number of shrews was observed both on the coast of the Sea of Okhotsk and in the continental part of Northeast Asia (the upper reaches of the Kolyma River) (Kiselev et al., 2014) indicates that this feature is common for most of the region. In any case, in the habitats preferred by S. caecutiens, i.e., larch forests, in the years of high abundance, shrews have a considerable impact on food items (at least, preferred ones). In both areas, shrews were caught using pitfall traps. It is known that these traps mainly catch nonresident individuals (Shchipanov et al., 2003). In this regard, it was previously assumed that the observed signs of inadequate feeding of shrews in the years of their high abundance could be a consequence of the difficulties in finding unoccupied territory by young dispersing animals (Kiselev et al., 2014). The lack of an home range could hinder stable foraging. The relationship between the abundance of shrews and invertebrates, revealed on the coast of the Sea of Okhotsk, however, suggests that not only nonresidents are subject to deterioration in nutrition in the years of high numbers of this mammals. The dependence of the fatness of shrews on the level of their abundance was also noted by other authors who used a different type of trap for capturing animals. In Western Finland (Norrdahl and Korpimӓki, 2002) and in the European part of Russia (Tolkachev, 2007), the body condition index (weight-to-length ratio) of common shrews caught with snap traps was inversely related to their population density. This, on the one hand, confirms that the deterioration in the feeding of shrews during the years of their high abundance is also characteristic of resident individuals; on the other hand, it indicates that this dependence is not unique for Northeast Asia. The high impact of this animals on the food base can be facilitated by the absence of specific behavior aimed at protecting home ranges. Shrews of the genus Sorex, apparently, do not protect their territory in summer (Oleinichenko, 2007, 2012; Shchipanov et al., 2019; Shchipanov, 2021). It is possible that social interactions of individuals, the frequency of which increases with the number of animals, can also affect the energy reserves of shrews and the intensity of their exploitation of food resources. Social contacts between con- and heterospecifics, a significant proportion of which are aggressive interactions (Kalinin et al., 1998; Kalinin and Shchipanov, 2003; Oleinichenko, 2007), are probably energetically expensive for shrews (Ochocińska and Taylor, 2005; Oleinichenko, 2007; Shchipanov, 2021). To maintain vital functions in conditions of increased energy expenditure, more food resources are needed, and therefore the effect on the food base during years with high abundance of animals can additionally increase.
The glycogen content in the liver of mammals is a quite variable parameter, depending on many factors. Experimentally, it was revealed that liver glycogen in shrews is reduced rather quickly during swimming in cold water (unpublished data). Animals that die in the traps with water thus have only residual amounts of glycogen. Nevertheless, these values are likely to correlate with those in the animals before falling into the traps. At the very least, the age and sex differences in the liver glycogen content in shrews caught by pitfall traps corresponded to those in live animals kept in captivity.
In the upper reaches of the Kolyma River, against the background of a decrease in the mass of fat reserves of shrews with an increase in their number, the level of glycogen in the liver, on the contrary, increased (Kiselev et al., 2014). It was assumed that this feature was a consequence of irregular feeding (an increase in the time intervals between meals) of shrews in the years of their high abundance. The content of glycogen in the liver in mammals is rather rapidly depleted during fasting; however, with prolonged access to high-calorie food after fasting, the level of this carbohydrate greatly increases and can be several times higher than normal (Mosin, 1982; Nur et al., 1995). A similar feature was noted in shrews (Kiselev, 2017b). On the coast of the Sea of Okhotsk, however, no relationship was found between the liver glycogen content and the abundance of S. caecutiens. The reason for the differences between the regions in the dynamics of the glycogen content of the liver of animals is not exactly clear; perhaps this is due to the differences in the abundance and composition of food items of shrews. In the examined part of the upper reaches of the Kolyma River, according to visual assessment, the biomass and diversity of invertebrates is higher than on the coast. The glycogen content in the liver and the mass of fat reserves in shrews from along the Kolyma River were also, in general, higher (Kiselev, 2017a). As noted above, only individuals who have received long-term access to high-calorie food after fasting are capable of accumulating an excessive amount of liver glycogen. This may not happen if, in years of high abundance, the shrews have difficulty finding nutritious food all the time.
The results of this and earlier studies indicate that, in Northeast Asia, the body condition of S. caecutiens decreases in the years of high abundance of shrews. The mass of fat reserves of the Laxmann’s shrew at the end of the summer period showed a positive relationship with the abundance of certain invertebrates. The relative biomass of food items, in turn, was inversely related to the abundance of shrews. Thus, the deterioration of the physiological state of shrews in the years of their high abundance is most likely a consequence of the increased competition for food resources. It is possible, however, that some other density-dependent factors, in particular, social interactions and competition for space, can also have a certain effect on the energy reserves of this animals. The mass of adipose tissue of S. caecutiens and the biomass of ground invertebrates in this study showed a correlation not so much with the abundance of this species, but with the abundance of all shrew species in aggregate, which may indicate not only intraspecific, but also interspecific competitive relationships.
The revealed features suggest that food competition is an important component of the density-dependent regulation of the shrew population. The greatest dependence on the abundance of shrews among invertebrates was shown by insect larvae, which apparently have a high nutritional value. Lack of nutritious food in conditions of increased energy expenditures for social interactions can contribute to high animal mortality.
REFERENCES
Bellocq, M.I. and Smith, S.M., Population dynamics and foraging of Sorex cinereus (masked shrew) in the boreal forest of eastern Canada, Ann. Zool. Fenn., 2003, vol. 40, no. 1, pp. 27–34.
Bellocq, M.I., Bendell, J.F., and Innes, D.G.L., Diet of Sorex cinereus, the masked shrew, in relation to the abundance of Lepidoptera larvae in northern Ontario, Am. Midl. Nat., 1994, vol. 132, no. 1, pp. 68–73.
Berman, D.I. and Bukhkalo, S.P., Population of soil-dwelling invertebrates of the sparse forest belt of the Upper Kolyma basin, in Poyas redkolesii verkhovii Kolymy (raion stroitel’stva Kolymskoi GES) (Sparse Forest Belt of the Upper Kolyma (Area of Construction of the Kolyma Hydroelectric Power Station)), Vladivostok: Dal’nevost. Nauchn. Tsentr Akad. Nauk SSSR, 1985, pp. 64–90.
Bobretsov, A.V., Lukyanova, L.E., Petrov, A.N., and Bykhovets, N.M., Periodic changes in the abundance of Laxmann’s shrew (Sorex caecutiens, Eulipotyphla) and factors of its population dynamics in the foothill taiga of the Northern Urals, Russ. J. Ecol., 2020, vol. 51, no. 3, pp. 282–287.
Buckner, C.H., Small mammals as predators of sawflies, Can. Entomol., 1955, vol. 87, no. 3, pp. 121–123.
Buckner, C.H., The common shrew (Sorex araneus) as a predator of the winter moth (Operophtera brumata) near Oxford, England, Can. Entomol., 1969, vol. 101, no. 4, pp. 370–375.
Bukhkalo, S.P., The functional and spatial structure of a population of soil-living invertebrates in the larch communities of Northeast Asia, Biol. Bull. (Moscow), 2009, vol. 36, no. 4, pp. 373–379.
Churchfield, S., Food availability and diet of the common shrew, Sorex araneus, in Britain, J. Anim. Ecol., 1982, vol. 51, no. 1, pp. 15–28.
Churchfield, S. and Sheftel, B.I., Food niche overlap and ecological separation in a multi-species community of shrews in the Siberian taiga, J. Zool. Lond., 1994, vol. 234, no. 1, pp. 105–124.
Churchfield, S., Hollier, J., and Brown, V.K., The effects of small mammal predators on grassland invertebrates, investigated by field exclosure experiment, Oikos, 1991, vol. 60, no. 3, pp. 283–290.
Churchfield, S., Nesterenko, V.A., and Shvarts, E.A., Food niche overlap and ecological separation amongst six species of coexisting forest shrews (Insectivora: Soricidae) in the Russian Far East, J. Zool. Lond., 1999, vol. 248, no. 3, pp. 349–359.
Davidson, M.B. and Berliner, J.A., Acute effects of insulin on carbohydrate metabolism in rat liver slices: independence from glucagon, Am. J. Physiol., 1974, vol. 227, no. 1, pp. 79–87.
Dokuchaev, N.E., Feeding of shrews (Soricidae) and assessment of their role in mountain-taiga ecosystems of North-Eastern Siberia, in Ekologiya mlekopitayushchikh Severo-Vostochnoi Sibiri (Ecology of Mammals of North-Eastern Siberia), Moscow: Nauka, 1981, pp. 3–22.
Dokuchaev, N.E., Seasonal changes in body weight in the Laxmann’s and Siberian large-toothed shrews in North-Eastern Siberia, Byull. Mosk. O-va Ispyt. Prir., Otd. Biol., 1983, vol. 88, no. 2, pp. 36–42.
Dokuchaev, N.E., Ekologiya burozubok Severo-Vostochnoi Azii (Ecology of Shrews of Northeast Asia), Moscow: Nauka, 1990.
Dokuchaev, N.E., Emelyanova, L.G., and Orekhov, P.T., Shrews of the Nadym River basin (north of Western Siberia), Contemp. Probl. Ecol., 2015, vol. 8, no. 1, pp. 51–55.
Fox, B.J. and Kirkland, G.L., Jr., An assembly rule for functional groups applied to North American soricid communities, J. Mammal., 1992, vol. 73, no. 3, pp. 491–503.
Genoud, M., Energetic strategies of shrews: ecological constrains and evolutionary implications, Mammal. Rev., 1988, vol. 18, no. 4, pp. 173–193.
Hanski, I. and Parviainen, P., Cocoon predation by small mammals, and pine sawfly population dynamics, Oikos, 1985, vol. 45, no. 1, pp. 125–136.
Henttonen, H., Haukisalmi, V., Kaikusalo, A., Korpimäki, E., Norrdahl, K., and Skaren, U.A.P., Long-term population dynamics of the common shrew Sorex araneus in Finland, Ann. Zool. Fenn., 1989, no. 26, pp. 349–355.
Holling, C.S., The components of predation as revealed by a study of small mammal predation of the European pine sawfly, Can. Entomol., 1959, vol. 91, no. 5, pp. 293–320.
Huitu, O., Norrdahl, K., and Korpimäki, E., Competition, predation and interspecific synchrony in cyclic small mammal communities, Ecography, 2004, vol. 27, no. 2, pp. 197–206.
Hyvärinen, H., Brown fat and the wintering of shrews, in Advances in the Biology of Shrews, Merritt, J.F., Kirkland, G.L., Jr., and Rose, R.K., Eds., Carnegie Mus. Nat. Hist. Spec. Publ., 1994, vol. 18, pp. 259–266.
Ivanter, E.V. and Makarov, A.M., Spatial organization of populations of shrews (Sorex, Insectivora) and its relationship with the feeding capacity of biotopes, Zool. Zh., 1994, vol. 73, no. 9, pp. 124–138.
Ivanter, E.V., Korosov, A.V., and Makarov, A.M., On the study of trophic relationships of small insectivorous mammals, Zool. Zh., 2015, vol. 94, no. 6, pp. 711–722.
Kaikusalo, A. and Hanski, I., Population dynamics of Sorex araneus and S. caecutiens in Finnish Lapland, Acta Zool. Fenn., 1985, no. 173, pp. 283–285.
Kalinin, A.A. and Shchipanov, N.A., Density-dependent behavior of shrews (Sorex araneus, S. caecutiens, and S. minutus) under natural and experimental conditions, Biol. Bull. (Moscow), 2003, vol. 30, no. 6, pp. 576–583.
Kalinin, A.A., Shchipanov, N.A., and Demidova, T.B., Behavior of four species of shrews Sorex isodon, S. araneus, S. caecutiens, and S. minutus (Insectivora, Soricidae) in interspecific contacts, Zool. Zh., 1998, vol. 77, no. 7, pp. 838–849.
Kalinin, A.A., Demidova, T.B., Oleinichenko, V.Yu., and Shchipanov, N.A., Seasonal dynamics of the number of shrews (Insectivora, Soricidae), Zool. Zh., 2008, vol. 87, no. 2, pp. 218–225.
Keicher, L., O’Mara, M.T., Voigt, C.C., and Dechmann, D.K.N., Stable carbon isotopes in breath reveal fast metabolic incorporation rates and seasonally variable but rapid fat turnover in the common shrew (Sorex araneus), J. Exp. Biol., 2017, vol. 220, part 15, pp. 2834–2841.
Kirkland, G.L., Jr., Competition and coexistence in shrews (lnsectivora: Soricidae), in The Biology of the Soricidae, Findley, J.S. and Yates, T.L., Eds., Albuquerque: Spec. Publ. Museum Southwestern Biol. Univ. New Mexico, 1991, pp. 15–22.
Kiselev, S.V., Physiological indicators of shrews in the Okhotsk-Kolyma country, in Chteniya pamyati akademika K.V. Simakova. Mater. dokl. Vseros. nauchn. konf. (Magadan, 22–24 noyabrya 2017 g.) (Academician K.V. Simakov Memorial Lectures: Proc. Vseros. Sci. Conf. (Magadan, November 22–24, 2017)), Magadan: IP Zharikova T.V., 2017a, pp. 143–146.
Kiselev, S.V., Physiological response in the even-toothed shrew Sorex isodon to fasting and refeeding, J. Evol. Biochem. Physiol., 2017b, vol. 53, no. 4, pp. 324–330.
Kiselev, S.V., Dynamics of the number and community structure of shrews in the surroundings of Magadan (northern coast of the Sea of Okhotsk), Contemp. Probl. Ecol., 2019, vol. 12, no. 5, pp. 464–472.
Kiselev, S.V. and Yamborko, A.V., Dynamics of the number of Laxmann’s shrew (Sorex caecutiens) and even-toothed shrew (Sorex isodon) populations in the Upper Kolyma river basin, Zool. Zh., 2014, vol. 93, no. 9, pp. 1106–1116.
Kiselev, S.V., Lazutkin, A.N., and Yamborko, A.V., Some physiological and biochemical parameters of underyearling Laxmann’s shrews (Sorex caecutiens Laxmann) and even-toothed shrews (Sorex isodon Turov) under different population densities, Biol. Bull. (Moscow), 2014, vol. 41, no. 1, pp. 71–79.
Klenovšek, T., Novak, T., Čas, M., Trilar, T., and Janžekovič, F., Feeding ecology of three sympatric sorex shrew species in montane forests of Slovenia, Folia Zool., 2013, vol. 62, no. 3, pp. 193–199.
Klyukin, N.K., Climate, in Sever Dal’nego Vostoka (North of the Far East), Moscow: Nauka, 1970, pp. 101–132.
Korolkova, G.E., Influence of shrews (Sorecidae) on invertebrates of forest litter and soil, in Materialy soveshchaniya “Rol’ zhivotnykh v funktsionirovanii ekosistem” (Proc. Conf. “The Role of Animals in the Functioning of Ecosystems”), Moscow: Nauka, 1975, pp. 140–143.
Korolkova, G.E., Small mammals of northern taiga biogeocenoses, in Osnovnye tipy biogeotsenozov severnoi taigi (Main Types of Biogeocenoses of Northern Taiga), Moscow: Nauka, 1977, pp. 260–269.
Makarov, A.M. and Ivanter, E.V., Dimensional characteristics of prey and their role in the diet of shrews (Sorex L.), Russ. J. Ecol., 2016, vol. 46, no. 3, pp. 315–320.
Moraleva, N.V., On the problem of interspecific relations of closely related species of shrews (Insectivora, Sorex), in Fauna i ekologiya ptits i mlekopitayushchikh Srednei Sibiri (Fauna and Ecology of Birds and Mammals of Central Siberia), Moscow: Nauka, 1987, pp. 213–228.
Mosin, A.F., Some physiological and biochemical features of starvation and refeeding in small wild rodents, Comp. Biochem. Physiol., Part A: Physiol., 1982, vol. 71, no. 3, pp. 461–464.
Namba, T. and Ohdachi, S.D., Top-down cascade effects of the long-clawed shrew (Sorex unguiculatus) on the soil invertebrate community in a cool-temperate forest, Mammal Study, 2016, vol. 41, no. 3, pp. 119–130.
Norrdahl, K. and Korpimӓki, E., Changes in individual quality during a 3-year population cycle of voles, Oecologia, 2002, vol. 130, no. 2, pp. 239–249.
Nur, T., Sela, I., Webster, N.J., and Madar, Z., Starvation and refeeding regulate glycogen synthase gene expression in rat liver at the posttranscriptional level, J. Nutr., 1995, vol. 125, no. 10, pp. 2457–2462.
Ochocińska, D. and Taylor, J.R.E., Living at the physiological limits: field and maximum metabolic rates of the common shrew (Sorex araneus), Physiol. Biochem. Zool., 2005, vol. 78, no. 5, pp. 808–818.
Oleinichenko, V.Yu., Behavior of underyearlings of the common (Sorex araneus), Laxmann’s (Sorex caecutiens), and Eurasian pygmy (Sorex minutus) shrews in familiar and foreign territories, Zool. Zh., 2007, vol. 86, no. 10, pp. 1259–1271.
Oleinichenko, V.Yu., Behavioral interactions of adult females of the common shrew (Sorex araneus) with conspecifics on familiar territory, Biol. Bull. (Moscow), 2012, vol. 39, no. 4, pp. 351–359.
Pernetta, J.C., Diets of the shrews Sorex araneus L. and Sorex minutus L. in Wytham grassland, J. Anim. Ecol., 1976, vol. 45, no. 3, pp. 899–912.
Shchipanov, N.A., Random processes and use of space in the common shrew (Sorex araneus L.), Russ. J. Ecol., 2021, vol. 52, no. 2, pp. 165–172.
Shchipanov, N.A., Kuptsov, A.V., Kalinin, A.A., and Oleinichenko, V.Yu., Pitfall traps and live traps catch different shrews (Insectivora, Soricidae), Zool. Zh., 2003, vol. 82, no. 10, pp. 1258–1265.
Shchipanov, N.A., Zima, J., and Churchfield, S., Introducing the common shrew, in Shrews, Chromosomes and Speciation, Searle, J. and Polly, P., Eds., Cambridge Studies in Morphology and Molecules: New Paradigms in Evolutionary Biology, Cambridge: Cambridge Univ. Press, 2019, pp. 19–67.
Sheftel, B.I., Long-term and seasonal dynamics of shrews in Central Siberia, Ann. Zool. Fenn., 1989, vol. 26, no. 4, pp. 357–369.
Shilov, I.A., Ekologiya (Ecology), Moscow: Vysshaya shkola, 1998.
Taylor, J.R.E., Evolution of energetic strategies in shrews, in Evolution of Shrews, Białowieza: Mammal Res. Inst. Pol. Acad. Sci., 1998, pp. 309–346.
Tolkachev, O.V., Vozdeistvie urbanizatsii na naselenie burozubok lesnykh ekosistem, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Yekaterinburg, 2007.
Volpert, Ya.L. and Shadrina, E.G., Melkie mlekopitayushchie severo-vostoka Sibiri (Small Mammals of Northeastern Siberia), Novosibirsk: Nauka, 2002.
Zakharov, V.M., Pankakoski, E., Sheftel, B.I., Peltonen, A., and Hanski, I., Developmental stability and population dynamics in the common shrew, Sorex araneus, Am. Nat., 1991, vol. 138, no. 4, pp. 797–810.
Zakharov, V.M., Demin, D.V., Baranov, A.S., Borisov, V.I., Valetsky, A.V., and Sheftel, B.I., Development stability and population dynamics of shrews Sorex in Central Siberia, Acta Theriol., 1997, vol. 42, suppl. 4, pp. 41–48.
ACKNOWLEDGMENTS
The author thanks N.E. Dokuchaev (Institute of Biological Problems of the North, Far East Branch, Russian Academy of Sciences) for help in determining the taxonomic affiliation of invertebrates, for information on the food preferences of shrews, and for comments on this work.
Funding
This study was supported by the Russian Foundation for Basic Research, project nos. 15-04-02668 and 18-04-00579.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The author declares that he has no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by N. Smolina
Rights and permissions
About this article
Cite this article
Kiselev, S.V. Interannual Variability in the Energy Reserves of Laxmann’s Shrew (Sorex caecutiens) on the Northern Coast of the Sea of Okhotsk. Biol Bull Russ Acad Sci 49, 107–116 (2022). https://doi.org/10.1134/S1062359022020121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359022020121