Abstract
The level of carboxyhemoglobin (CO-Hb) in the forensic blood sample is a critical evidence to the conclusion that the fatality was related to CO poisoning or other reason causing death. In this study, we validated the method for CO-Hb determination in blood using headspace gas chromatography−flame ionization detector (HS-GC/FID) for further application to the analysis of forensic samples. The liberated CO from CO-Hb was reduced to methane by a specific catalyst and then analyzed by GC−FID. The quantitative range for CO-Hb was from 2.5 to 100% with the limit of detection down to 0.5%. The average recovery at different levels of CO-Hb was from 96.8 to 98.5% with high repeatability and intermediate precision. The obtained parameters presented that the method was highly sensitive and accurate for CO-Hb determination in blood samples. The method was also applied to the analysis of 27 forensic samples that had the questionable cause of death. Eighteen samples were related to CO poisoning with the level of CO-Hb exceeding 15%. All the results demonstrated that this method could be applied for forensic diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Carbon monoxide is a chemical produced from incomplete burning of natural gas or other products containing carbon, including exhaust, faulty heaters, fires, and factory emissions [1, 2]. This odorless gas is the leading cause of poisoning deaths in many countries in the world [3, 4]. Carboxyhemoglobin (CO-Hb) level in healthy non-smoking subjects is less than 2% and less than 15% for smokers. At CO-Hb levels below 10%, no notable symptoms are observed. Neurological symptoms such as nausea, headache, and dizziness are observed at CO-Hb levels over 10%. An increase in respiratory and heart rates, syncope, motor paralysis, and confusion are observed at CO-Hb levels of 30–50%. CO-Hb levels exceeding 50% are considered as life-threatening, and values in this range are central to the diagnosis of CO poisoning [5–8]. Although for almost all fatal cases caused by CO poisoning statistics are obtained annually in most countries, some of them were grossly reported without CO-Hb quantitation in blood. Therefore, it is difficult to know the exact number of fatalities caused by CO poisoning.
The quantitation of CO-Hb in blood is an essential step to confirm that CO poisoning or another reason causes death. The concentration of CO-Hb would be much higher than 15% if the death was related to CO poisoning. Therefore, the determination of the exact cause of death from CO poisoning is currently of concern in forensic examination.
The spectrophotometric method is the most widely used for the evaluation of CO-Hb presence. This method is based on the changes in absorption spectrum [9–11]. However, the sensitively may still be the limitation of this method. Some other methods have also been applied for CO-Hb determination such as detection tubes [12, 13], oximetry [14], or fluorescent sensor [15]. Despite the successes of these methods, their limited sensitivity and time-consuming analysis may still be the drawbacks of these applications. Airtight gas syringe−gas chromatography−mass spectrometry (AGS−GC−MS) also requires expensive instruments and is time consuming [16]. Recently, a number of methods based on gas chromatography have been reported to determine CO in blood. The detectors used in these methods include thermal conductivity detector [17, 18], barrier discharge ionization detector (BID) [19], and flame ionization detector [20, 21]. The most advantageous method currently used is GC−FID. Focusing on this point, this study aims to validate a sensitive method for CO-Hb determination in blood using headspace gas chromatography−flame ionization detection (HS−GC/FID) for further application to the analysis of forensic samples.
By using the specific catalytic reduction of released CO from CO-Hb to methane and then detection by a flame ionization detector [22], the method promises high specificity and sensitivity in CO determination. Furthermore, the merits of the method were observed based on the real blood sample analysis, showing a highly accurate method that can be applied for diagnostic and forensic examination.
EXPERIMENTAL
Materials and reagents. Saponin (lot #BCC3326) and octanol (>99%) were purchased from Sigma-Aldrich (Singapore). CO (>99.99%) and N2 (>99.99%) were supplied from Viet Nguyen Technology Service Trading Co. (Ho Chi Minh City, Vietnam). All other reagents of analytical grade used in this work were purchased from Merck (Merck, Darmstadt, Germany). Lithium heparin tubes were supplied from Hong Thien My Joint-stock Co. (Ho Chi Minh City, Vietnam). All solutions were prepared using ultra-pure water (with an electrical resistivity >18.3 MΩ cm) produced by a Millipore Milli Q system (Billerica, MA, USA). The blank blood samples used in this study were collected from the non-smoking volunteers. The real samples were collected from the Forensic Medicine Centre of Ho Chi Minh City (Vietnam).
Calibration curve preparation and sample analysis. The calibration curves and sample analysis were prepared as follows: the collected blood sample from volunteers was anticoagulated and stored in lithium heparin tubes. To 8 mL of blood, 100 µL of antifoaming agent, n-butanol, were added and then purged with N2 at the flow rate of 5 mL/min for 5 min. The resulting mixture was then rotated for 15–30 min. The final obtained mixture was 0% CO-Hb blood standard. The 100% CO-Hb blood standard was prepared similarly, except purging with CO at 10 mL/min for 30 min instead of N2. Both mixtures were stored at 4°C for daily use.
Each standard point of the calibration curve was prepared by mixing the 0 and 100% CO-Hb blood standards with a certain volume ratio (the final volume was 400 µL). CO from CO-Hb was liberated by incubating this mixture with 800 µL of saponin−H2SO4 (7.5 g of saponin in 473 mL of H2O and 27 mL of 98% H2SO4) at 100°C for 45 min. The extracted CO was then reduced to methane by nickel catalyst kit (part number: G3478A) at 375°C with a hydrogen rich atmosphere. The produced methane was later determined by GC−FID.
The real forensic blood samples were prepared by the following CO liberating steps: 400 µL of fresh blood was incubated with 800 µL of saponin in H2SO4 at 100°C for 45 min in the headspace vial. The released CO was transformed into methane and was detected by GC−FID under operating conditions shown in Table 1.
RESULTS AND DISCUSSION
Specificity of the method. The accuracy of the method for determining CO in blood was verified by the specificity, linearity, limit of detection (LOD), recovery, repeatability, intermediate precision, and uncertainty measurement. These merits were validated according to the guidance in AOAC 2016, ANSI/ASB 072-2019 [23–25]. The specificity of CO detection was observed by analysis of blank matrix and spiked samples. The chromatograms of the blank sample and samples spiked with CO-Hb at different levels are shown in Fig. 1.
For the blank matrix, a small peak at the retention time of 3.92 min was observed in the chromatogram. The peak area was about 1.71 (Fig. 1a) demonstrating that there was a small amount of CO-Hb in the blank blood sample. In contrast, when analyzing the matrix containing 1.5% CO-Hb, the signal obviously changed for the peak at 3.92 min with a peak area of 11.27 (Fig. 1b). The signal ratio compared with the blank sample was 6.61. To further confirm the specificity of the CO-Hb peak, another spiked sample was analyzed with CO-Hb amount of 20%. The obtained peak at 3.91 min had the peak area of 204.77 (Fig. 1c), and the area ratio to blank sample was 120.1. All these results confirmed the high specificity of the signal in CO-Hb detection mediating the high specificity of CO-Hb determination in the blood sample.
Linearity of the method. In order to minimize matrix effect to the analytical signal, the matrix-matched calibration was used to observe linearity of the method. For each calibration curve, nine standard points with various CO-Hb concentrations were observed. The calibration curves were constructed for three days with six repeated experiments (n = 6). As shown in Table 2, CO-Hb in blood can be determined in the range of 2.5 to 100% with the correlation coefficient (R2) of 0.999. The stability of calibration curves is presented by the high correlation coefficient (0.999) for three days. The observed linearity of the method confirmed that this method could be applied for CO determination in the blood sample.
Method detection limit and quantitation limit. As there was a small peak of CO-Hb in the blank matrix (Fig. 1a), the detection limit was evaluated based on the analysis of the blank sample. Ten repetitive experiments were carried out for three different blank samples. LOD of the method was calculated as the average concentration of CO-Hb in the blank matrix plus three times the standard deviation (SD), and the limit of quantification (LOQ) was the average concentration plus ten times the SD [22]. LOD for CO-Hb determination was 0.5%, and LOQ was 1.1% (Table 3). According to the poisoning level of CO-Hb, the LOD and LOQ values showed that the method was very sensitive for CO-Hb determination in blood samples.
Accuracy and precision of the method. Due to the lack of the certified reference materials, the trueness was evaluated by method recovery (intra-laboratory reproducibility). These three samples spiked with CO-Hb at 25, 50, and 90% were analyzed on three different days. The recovery of CO-Hb was from 93.8 to 98.5% (Table 4). These recoveries had the same value of 100 at 95% confidence (one-sample t-test). The recovery was stable on different days and with different analysts with intermediate precision expressed as the relative standard deviation (RSD) lower than 5.9%. The method showed high repeatability in six repetitive analyses of three spiked blood samples. At CO-Hb concentration of 25, 50, and 90%, the relative standard deviations observed were 5.7, 4.8, and 4.2%, respectively.
The measurement uncertainty of the method was also validated based on “top-down” approach [26]. The uncertainty was calculated from the calibration curve, repeatability, intermediate precision, and bias (percent recovery). The obtained measurement uncertainty was 8.2%, confirming the high accuracy of CO-Hb determination in blood samples.
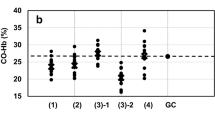
Practical application of the method. The validated method was used to analyze 27 forensic blood samples collected from the Forensic medicine Centre of Ho Chi Minh City. The level of CO-Hb in blood samples was in the range of 1–67%. The results were classified by concentration and are presented in Fig. 2. In 27 analyzed samples, the number of samples with CO-Hb concentration below 15%, 15–50%, and higher than 50% CO-Hb was 9, 12, and 6, respectively. There were 18 cases of CO poisoning with CO-Hb concentration higher than 15% (equivalent to 67%). One-third of the cases were heavy poisoning with CO-Hb level exceeding 50%. The remain fatal cases, where the concentration was lower than 15%, were not caused by CO poisoning (equivalent to 33%). The results are shown in Fig. 2.
CONCLUSIONS
An efficient method has been validated for the quantification of CO-Hb in blood samples. The method was based on the specific catalysis to reduce released CO from CO-Hb to methane followed by analysis using gas chromatography with a flame ionization detector. The method expressed high specificity and selectivity in CO-Hb determination in blood. All evaluated merits proved that the method was suitable for quantitative analysis. The validated method was applied to the analysis of 27 forensic blood samples. The results showed that there were 67% of sample cases related to CO poisoning. This work supplied an efficient method for CO-Hb determination in blood samples with high accuracy and can be applied for analyzing forensic samples.
REFERENCES
Almirall, J.R. and Furton, K.G., J. Anal. Appl. Pyrolysis, 2004, vol. 71, p. 51.
Karjalainen, P., Pirjola, L., Heikkila, J., Lahde, T., Tzamkiozis, T., Ntziachristos, L., Keskinen, J., and Ronkk, T., Atmos. Environ., 2014, vol. 97, p. 262.
Sircar, K., Clower, J., Shin, M., Bailey, C., King, M., and Yip, F., Am. J. Emerg. Med., 2015, vol. 33, p. 1140.
Hosseininejad, S.M., Aminiahidashti, H., Khatir, I.G., Ghasempouri, S.K., Jabbari, A., and Khandashpour, J. Forensic Leg. Med., 2018, vol. 53, p. 87.
Prockop, L.D. and Chichkova, R.I., J. Neurol. Sci., 2007, vol. 262, p. 122.
Raub, J.A., Mathieu-Norf, M., Hampson, N.B., and Thom, S.R., Toxicology, 2000, vol. 145, p. 1.
World Health Organization. International Programme on Chemical Safety, Environmental Health Criteria 213: Carbon Monoxide, Geneva, 1999, 2nd ed.
Powers, R.H. and Dean, D.E., Pulmonary Toxicology, Forensic Toxicology Mechanisms and Pathology, Boca Raton: CRC, 2016, p. 147.
Katusmata, Y., Aoki, M., Sato, K., Suzuki, O., Oya, M., and Yada, S., J. Forensic Sci., 1982, vol. 27, p. 928.
Fukui, Y., Matsubara, M., Takahashi, S., and Matsubara, K., J. Anal. Toxicol., 1984, vol. 8, p. 277.
Ohmori, T., Saito, Y., Mamiya, K., Sasaoka, S., Suzuki, Y., Namekawa, Y., Otsuka, K., Kogure, S., Mochizuki, A., Nomura, Y., Asaoka, K., Saito, T., Yoshida, K., Ojima, M., Koizumi, T., Kumihashi, M., Shimada, H., Wakita, S., Otsuka, M., and Seto, Y., Forensic Toxicol., 2019, vol. 37, p. 330.
Akane, A. and Fukui, Y., Res. Rep. Forensic Med. Sci., 1985, vol. 28, p. 185.
Sato, K., Carbon monoxide, in Drugs and Poisons in Humans: A Handbook of Practical Analysis, Suzuki, O. and Watanabe, K., Eds., Berlin: Springer, 2005, p. 91.
Zaouter, C. and Zavorsky, G.S., Respir. Physiol. Neurobiol., 2012, vol. 182, p. 88.
Shi, G., Yoon, T., Cha, S., Kim, S., Yousuf, M., Ahmed, N., Kim, D., Kang, H.W., and Kim, K.S., ACS Sensors, 2018, vol. 3, p. 1102.
Oliverio, S. and Varlet, V., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2018, vol. 1090, p. 81.
Lewis, R.J., Johnson, R.D., and Canfield, D.V., J. Anal. Toxicol., 2004, vol. 28, p. 59.
Seto, Y., Kataoka, and Tsuge, M.K., Forensic Sci. Int., 2001, vol. 121, p. 144.
Ohmori, T., Otsuka, M., and Seto, Y., Rep. Natl. Res. Inst. Police Sci., 2017, vol. 66, p. 59.
Guillot, J.G., Weber, J.P., and Savoie, J.Y., J. Anal. Toxicol., 1981, vol. 5, p. 264.
Czogała, J., Wardas, W., and Goniewicz, M.L., Anal. Chim. Acta, 2006, vol. 556, p. 295.
Walch, S.G., Lachenmeier, D.W., Sohnius, E.M., Madea, B., and Musshoff, F., Open Toxicol. J., 2010, vol. 4, p. 21.
Guidelines for Standard Method Performance Requirements, AOAC Official Methods of Analysis, Appendix F, AOAC International, 2016. http://www. eoma.aoac.org/app_f.pdf. Accessed March 31, 2016.
ASB Standard 036: Standard Practices for Method Validation in Forensic Toxicology, USA: Academy Standards Board, 2017.
ANSI/ASB Standard 072: Standard for the Validation of Procedures in Bloodstain Pattern Analysis, USA: AAFS Standards Board, 2019.
EURACHEM/CITAC Guide CG4: Quantifying Uncertainty in Analytical Measurement, Ellison, S.L.R and Williams, A., Eds., 2012, 3rd ed. https://www.eurachem.org/index.php/publications/guides/quam. Accessed March 30, 2019.
Funding
The authors would like to thank the Industrial University of Ho Chi Minh City for financial supports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Le, DV., Dang, MT. Application of Headspace Gas Chromatography with Flame Ionization Detector for Determination of Carboxyhemoglobin in Forensic Blood Samples. J Anal Chem 76, 1430–1434 (2021). https://doi.org/10.1134/S1061934821120042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934821120042