Abstract
A method for the quantification of a mixture of fluorescent antibiotics with broad, highly overlapped excitation and emission spectra is proposed. The method enables the simultaneous determination of sarafloxacin, flumequine and ofloxacin by using the fluorescence excitation-emission matrix and performing two different scans, namely, a linear variable angle synchronous fluorimetry scan at 15.6° and an emission scan at λexc of 320 nm. This ensures complete resolution of the mixture without the need for a separation technique. Various factors influencing the fluorescence intensity were assessed in order to maximize the sensitivity and obtain an adequate selectivity. The method was successfully used to determine the analytes in pharmaceuticals and rabbit plasma. The results obtained compared favorably with those provided by HPLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The majority of quinolones of clinical use belong to the subset of fluoroquinolones which have a fluoro group attached to the central ring system (typically at the 6-position). Fluoroquinolones are widely used in human and veterinary medicine [1, 2] because they can enter cells easily, thereby facilitating treatment of intracellular infections. In fact, these compounds are extremely effective in the treatment of a variety of infections including urinary tract infections, soft tissue infections, respiratory infections, bone-joint infections, typhoid fever, sexually transmitted diseases, prostatitis, community acquired pneumonia, acute bronchitis and sinusitis. However, fluoroquinolones should be employed judiciously because inappropriate use is likely to worsen current problems with antibiotic resistance.
The clinical and pharmaceutical determination of these drugs requires effective analytical methods for quality control as well as pharmacodynamic and pharmacokinetic studies. Developing and validating such methods is crucial with a view to optimizing the quantification of drugs by the pharmaceutical industry and to assuring quality of the end-product. Quinolones have so far been determined by using various techniques including atomic absorption spectrometry [3], capillary electrophoresis (CE) [4, 5], flow injection analysis [6], spectrofluorimetry [7, 8], HPLC [9, 10], liquid chromatography (LC) [11, 12], solid-phase extraction (SPE)−LC [13], mass spectrometry [14–16], tandem mass spectrometry [17], luminescence spectroscopy [18], voltammetry and polarography [19, 20], as well as specific spectrophotometric [21, 22] and titrimetric methods [23].
Chromatographic methods for quinolones are extensively used and endorsed. However, they generally require sophisticated, expensive equipment, provision for use and disposal of solvents, labor-intensive sample preparation procedures and skills in operating chromatographic techniques.
Spectrofluorimetry is widely used as a quantitative analytical tool in various areas. Also, fluorescence-based methods have gained increasing recognition for their excellent sensitivity, selectivity, non-invasiveness and expeditiousness. However, successful analysis of a fluorescent multicomponent system containing compounds with overlapped fluorescence bands without separation or derivatization is impossible. These complex samples require fluorimetric methods with improved selectivity such as those based on synchronous [24] or derivative [25] fluorescence spectrometry.
The analysis of multicomponent mixtures using conventional excitation or emission spectra measured at a single emission or excitation wavelength, respectively, is usually very difficult but can be facilitated by measuring a set of fluorescence spectra recorded at different excitation wavelengths (λexc). This allows a three-dimensional landscape called an “excitation–emission matrix” (EEM) to be obtained for each sample. The main advantage of EEMs is that they contain more information about fluorescent species than conventional excitation and emission spectra because they include emission bands excited at variable wavelengths potentially corresponding to different emissive species. EEMs can be represented as level curves or contour maps. The inception of computers in the laboratory has enabled the production of any type of spectra from EEMs [26]. Choosing an appropriate trajectory allows spectral resolution to be increased and/or the intensity of a particular fluorescent component to be selectively enhanced.
Synchronous fluorescence spectrometry (SFS) is a modification of the conventional fluorescence technique that provides improved selectivity through narrower spectral bands and simplified spectra. There are various SFS modes. One is variable angle synchronous fluorimetry (VASF) in which the difference between the emission and excitation wavelengths is continuously changed across a scan. This technique which is obviously more selective than SFS and also quite flexible was developed by Kubic et al. [27]. A great difficulty in applying the synchronous technique is that the best route must be known beforehand for optimum results. However, the best route can be readily determined from a three-dimensional plot allowing a total luminescence spectrum to be previously obtained in order to fully characterize the fluorescent properties of the sample. This technique affords identification of the specific paths yielding the strongest signals; also, it usually provides very good results because it overcomes energy transfer processes by circumventing “conflictive” spectral areas. However, it does not ensure total resolution of multicomponent mixtures of strongly overlapped compounds which usually require using various routes for this purpose.
Derivative fluorescence spectrometry differentiates a fluorescence spectrum by using a mathematical expression to transform the spectral curve into a derivative. Combining synchronous and derivative techniques results in increased sensitivity and selectivity by effect of the decreased spectral bandwidth relative to the emission spectrum and the amplitude of the secondary spectral signal which is easily masked by the dominant signal. The combined technique which usually provides better defined fingerprints than traditional spectrofluorimetry and affords discrimination from broadband interferents has been extensively used for the simultaneous determination of compounds with overlapping spectra in mixtures [28–30] and plays a prominent role in the multicomponent analysis of mixtures.
This paper reports a method for the quantification of a mixture of fluorescent antibiotics with broad, highly overlapped excitation and emission spectra. The method improves the simultaneous determination of sarafloxacin (SARA), flumequine (FLU) and ofloxacin (OFLO) by using fluorescence excitation–emission matrices and two different routes for complete resolution, namely, a variable angle synchronous fluorimetry scan and an emission scan. The proposed method requires simpler instrumentation, is less time-consuming and more sensitive than its conventional counterpart and has good selectivity. It was successfully used to determine the three target fluoroquinolones in pharmaceuticals and in rabbit plasma.
EXPERIMENTAL
Apparatus. All fluorimetric measurements were made on an Aminco Bowman series 2 luminescence spectrometer (Thermo Fisher Scientific, USA) governed via the software AB2 for the OS2 operating system. The instrument was equipped with a continuous 150 W Xenon lamp, Czerny-Turner monochromators of 200 mm focal distance and 1200 lines/mm holographic gratings, adjustable slits on both monochromators and a gated photomultiplier. Quartz glass cuvettes of 1.0 × 1.0 cm light path were used throughout the experiment.
Thermostating equipment, a Crison model 2001 pH-meter (Barcelona, Spain) equipped with a glass-saturated calomel combination electrode and a Mixtaxel Selecta centrifuge (Barcelona, Spain) were also used.
The software AB2 affords file management and setting instrumental parameters for acquisition of excitation and emission spectra.
Software. The software Ftotal [26] was used for optimal characterization of each fluorescent compound from a total luminescence spectrum. Three-dimensional spectra can be obtained and presented as contour maps. The software affords autoscaling and suppression of light scatter, which allows the fluorescent properties of target compound to be exploited in full. Besides, it processes the spectral data to obtain any type of bidimensional spectra. For the VASF spectrum, Ftotal software obtains the intensity values by applying Lagrange’s interpolation method to the excitation wavelengths calculated by means of the particular function that relates emission wavelengths at 0.8 nm intervals with the excitation wavelengths since they are not equidistant.
Reagents and solutions. All experiments were performed with analytical reagent grade chemicals, pure solvents and Milli-Q water. Sarafloxacin, flumequine and ofloxacin were obtained from Sigma-Aldrich (Steinheim, Germany). 50 mg/L stock solutions of each analyte were prepared in amber-colored flasks by dissolving appropriate amounts in water. The stock solutions were used on daily basis to prepare working solutions. A 1 M buffer solution at pH 3.0 was prepared by mixing appropriate amounts of sodium monochloroacetate and monochloroacetic acid. All stock standard solutions were stable at room temperature for at least 1 month, and the working samples of each compound for at least 2 h.
Pharmaceutical preparations, Ofloxacino Teva (Teva Genéricos, Madrid, Spain) and Flumesyva (Laboratorio Syva, Leon, Spain), were bought in a local drugstore.
Lyophilized plasma of rabbits was obtained from Becton, Dickinson and Company (Sparks, MD USA). It was reconstituted in each vial with 3 mL of purified water and kept at 4°C until use.
Procedures. Sample preparation. Calibration graphs were constructed by placing an aliquot of SARA, FLU and OFLO equivalent to an amount of 0.15–2.5, 0.5–6.5 and 0.15–2.5 µg, respectively, in a 25 mL volumetric flask, adding 1.5 mL of buffer solution (pH 3.0) and diluting with water to a final volume of 25 mL.
Each sample was used to record 61 emission spectra 384 nm wide in steps of 0.8 nm by changing the excitation wavelength in 6.4 nm steps. Total luminescence spectra were recorded by using the software Ftotal [26]. Then, the function passing through the selected excitation and emission maxima for SARA and FLU was used to obtain the VASF spectrum and a horizontal cut (λexc of 320.0 nm) equivalent to an emission spectrum. The first derivative of the spectrum was calculated by using the Savitzky–Golay algorithm [31, 32]. The absolute value of the derivative of the synchronous spectra at λem of 496.8 nm and λexc of 291.7 nm for SARA, λem of 344.0 nm and λexc of 249.1 nm for FLU and the emission scan at λem of 460.0 nm for OFLO were used for their determination.
Procedure for pharmaceutical samples. A powdered tablet of Ofloxacino Teva was placed in a 1 L volumetric flask, the volume of water needed to dissolve it was added and the mixture was shaken and kept in an ultrasonic bath for 15 min after which the flask was made to 1 L with water and the solution was filtered through paper for quantitative analysis (DP 145 125 from Albet). The filtered solution containing 100 mg/L OFLO was placed in a 1 L volumetric flask. In parallel, 1 g of Flumesyva water soluble powder was weighed, dissolved in water, filtered and placed in a 1 L volumetric flask that was made to the mark with water to obtain a solution containing 100 mg of FLU per 1 L.
Three different samples of each drug were prepared and then diluted as needed to bring their concentrations within calibration range. The resulting solutions were used as unknown samples and their concentrations were predicted under the optimized chemical and instrumental conditions.
Procedure for rabbit plasma. In order to accurately model plasma interactions, reconstituted rabbit plasma was spiked with appropriate amounts of OFLO, SARA and FLU to prepare synthetic standards. Aliquots from the standards were then transferred into calibrated flasks and the general procedure was applied. Fluoroquinolone contents were determined by using the standard addition method.
RESULTS AND DISCUSSION
Spectral features. The fluorescence of the compounds was optimally characterized from a three-dimensional spectrum. The spectrum was an isometric projection obtained by recording and plotting several excitation spectra at stepped increments of emission wavelength. A reversed projection of the data (i.e., one where the emission spectra were plotted at decreasing excitation wavelengths) can occasionally expose emission peaks hidden by the foreground. Alternatively, three-dimensional spectra for unknown samples can be effectively transformed into two-dimensional plots of the excitation and emission wavelengths by linking points of the same intensity to construct contours. In general, contour plots are more useful than isometric projections to reveal hidden emission peaks; also, they afford selection of the optimum trajectory for the application of the synchronous scan technique.
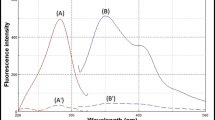
Figure 1 shows the total fluorescence spectra for OFLO, SARA and FLU recorded under the following conditions: slit width—4 nm, voltage—600 V, scan rate—50 nm/s and analyte concentration—500 ng/mL. We used three-dimensional emission spectra because their maximum emission wavelengths are slightly more different than are excitation wavelengths. Figure 1 shows the three-dimensional spectra for the analytes, where Rayleigh scattering has been removed. As can be seen, each analyte exhibited two excitation bands, namely, SARA at λexc1 of 280.0 nm and λexc2 of 324.0 nm (λem of 452.0 nm), FLU at λexc1 of 254.0 nm and λexc2 of 324.0 nm (λem of 363.0 nm), OFLO at λexc1 of 290.0 nm and λexc2 of 333.0 nm (λem of 486.0 nm).
The total fluorescence spectra for SARA, FLU and OFLO are shown as contour lines in Fig. 2. As can be seen, spectral overlap precluded the simultaneous determination of the three compounds by conventional spectrofluorimetry at their wavelength maxima.
Influence of experimental variables. Chemical variables were optimized to obtain the best measurement conditions, maximum fluorescence sensitivity and adequate selectivity.
The influence of pH on the fluorescence intensity was examined by adding different amounts of HCl and NaOH to the mixture. The fluorescence intensity of OFL increased with increasing pH to 3.0, above which it decreased; this was followed by an increase above pH 4.5, a peak at pH 7 and a new sharp decrease at higher values. The variation of the fluorescence intensity of OFLO with pH was accompanied by a change in the location of its spectral maximum (specifically, a bathochromic shift in the excitation and emission wavelengths). The fluorescence intensity of SARA increased with increasing pH to 6.0 and then decreased to zero in basic media; also, the maximum underwent a hypsochromic shift in the excitation and emission wavelengths. Finally, the fluorescence intensity of FLU remained constant up to pH 7.4, above which it decreased to zero. Based on these results, pH 3.0, adjusted with 0.06 M sodium monochloroacetate/monochloroacetic acid buffer, was used to determine the three analytes because it led to an increased fluorescence intensity for FLU which was the fluoroquinolone with the lowest quantum yield.
The influence of temperature on the fluorescence intensity of OFLO, SARA and FLU was examined from 4 to 35°C. As expected, a rise in temperature caused a slight decrease in fluorescence intensity for the three compounds. Therefore, temperature control was needed. A temperature of 20°C was chosen because it was close to room level.
The concentration range over which the fluorescence intensity of each analyte responded linearly was established by using solutions containing increasing concentrations of the analytes that were measured under the above-described optimized conditions. Linearity was lost at fluoroquinolone concentrations greater than 500 ng/mL.
Performance of the proposed method. Selection of the analytical signals. The simultaneous determination of the three fluoroquinolones required using synchronous fluorescence. The greatest difficulty in applying the synchronous scan technique is that the best route must be known before in order to ensure optimum results. Careful inspection of the contour maps was therefore required. The optimum route was immediately obvious even to a relatively inexperienced operator.
The optimum synchronous scan route was identified by carefully examining the contour map for a standard solution of the three compounds at concentration levels resulting in the same fluorescence intensity. The selected route was optimized in order to minimize spectral interferences with no loss of sensitivity.
As can be seen from the contour plots in Fig. 2, the spectra for the three compounds were clearly overlapped. This precluded their simultaneous determination with any synchronous fluorescence spectrometry technique not even in derivative form. This problem was overcome by using the derivative technique with two different scan routes. The combination of the two routes allowed SARA and FLU to be quantified in the presence of OFLO by linear variable angle synchronous fluorescence spectrometry (LVASF) in derivative form. On the other hand, OFLO in the ternary mixture was determined from the derivative of its emission spectrum.
Based on the contour plots, the optimum route was one passing by the excitation and emission maxima of SARA (λem1 of 362.4 nm, λexc1 of 254.3 nm) and FLU (λem2 of 460.0 nm, λexc2 of 281.5 nm). This route corresponded to an LVASF scan at 15.6° (see Fig. 2). The optimum route for OFLO (Fig. 2) was that passing by λexc1 of 320.0 nm in the emission scan.
Simultaneous determination of the three substances. Figure 3 shows the LVASF spectra for SARA, FLU and OFLO. The strong overlap in the spectra precluded the simultaneous determination of the three analytes by LVASF alone. Figure 4 shows the first derivatives of their LVASF spectra. Although the bands were not fully resolved, using the zero-crossing technique allowed acceptable results for SARA and FLU in the presence of OFLO to be obtained by using λem of 344.0 nm and λexc of 249.1 nm for FLU as well as λem of 496.8 nm and λexc of 291.7 nm for SARA. This procedure, however, did not allow OFLO to be quantified with adequate sensitivity owing to the absence of a wavelength with a zero fluorescence intensity for FLU and SARA. Instead, we used an emission spectrum recorded at λexc of 320.0 nm (Fig. 5), where overlap between OFLO and the two fluoroquinolones was less marked and allowed OFLO to be quantified from its first derivative spectrum by using the zero-crossing technique (see Fig. 6).
The optimum derivative spectra in terms of signal-to-noise ratio were obtained by using 25 points.
Statistical results. Mutual independence of the analytical signals for SARA, FLU and OFLO (i.e., independence of the selected zero-crossing wavelengths of the compounds present) was checked by constructing three calibration graphs from the first derivative signal for each analyte in the absence and presence of variable concentrations of the others. The concentration ranges spanned by the calibration graphs and the concentrations of the other compounds are given in the second and third columns, respectively, of Table 1.
The absence of outliers which can result in errors in the true line when experimental data are fitted by least squares regression was checked by using least median of squares regression (LMS) [33] which is robust enough for this purpose. No outliers were detected.
The analytical performance of the proposed method was assessed by subjecting the experimental results to a statistical analysis involving fitting the least squares line according to an equation of the type y = a + bx [34, 35]. Table 1 shows the figures of merit of the analysis.
The most representative calibration graph was identified by conventional overall least squares regression of all data pairs. The accuracy of the overall regression was assessed with an analysis of variance (ANOVA) test comparing the three lines for each analyte [35]. F1 values compare total deviations from the overall linear region with the deviation within each set from the set line in order to assess the suitability of the single overall regression line. If the experimental F1 value is less than its theoretical counterpart, then the deviation of the individual sets from the overall regression line is not significant. F2 values compare the differences between regression coefficients (i.e., the slopes with the deviation within each set from the set line testing the differences between regression coefficients). If the experimental F2 value is less than its theoretical counterpart, then no significant differences exist between the individual slopes and the overall regression slope can be assumed to be representative. The overall regression lines were all accurate, as confirmed by the fact that the experimental values of F1 and F2 were less than the theoretical values at the 95% confidence level (Table 1).
The residuals of the regression lines exhibited uniform variance (measurement errors were independent of the drug concentrations); therefore, the regression lines met the homoscedasticity requirement [34, 35].
The significance of the intercept on the y-axis was assessed from the 95% joint confidence intervals for the parameters of the linear regression model [35]. If the intercept (a) is plotted against the slope (b) for repeated random samples, the points will fall elliptically around the true center (α, β); conversely, any confidence interval for the true combination of α and β will take the form of an elliptical region around the best estimates (a, b) as center. If the zero intercept falls within the joint confidence region, then the intercept is not significantly different from zero and a proportional relationship between the analytical signal and concentration can be assumed. All intercepts were not significantly different from zero.
The detection limits (LOD) for SARA, FLU and OFLO determined according to IUPAC [36, 37] (i.e., as three times the standard deviation of the blank) were 0.6, 0.6 and 0.4 ng/mL, respectively. According to IUPAC, propagation errors lead to a detection limit consistent with the reliability of blank measurements and signal measurements of the standards [38, 39]; the detection limits thus obtained were 2.9, 3, and 1.9 ng/mL for SARA, FLU and OFLO, respectively. Finally, the criterion of Clayton [40] is based on the probability of positive false positives and negatives; its application led to the detection limits of 6, 5, and 4 ng/L for SARA, FLU and OFLO, respectively. These last values include all potential types of error and are probably thus the most accurate.
The precision of the method was assessed by using ten solutions containing 55, 200, and 60 ng/mL SARA, FLU and OFLO, respectively. The absolute values of the first derivative scan for the analytes were measured at 496.8, 344.0, and 460.0 nm, respectively. The resulting relative standard errors calculated in accordance with the error propagation criterion at the 95% confidence level were 3.4, 0.9, and 2.8% for SARA, FLU and OFLO, respectively, and the respective relative standard deviations (RSDs) for the replicates were 1.9, 2.1, and 1.2%.
The simultaneous determination of SARA, FLU and OFLO was validated by using least squares regression [35] to compare the proposed method with a chromatographic method [41]. Ten samples containing the studied compounds at concentrations over the application range were analyzed with the two methods. A least squares paired analysis was done [34] with the concentration calculated in reverse with both. If the two led to identical concentrations in each sample, then the least squares analysis would give a zero intercept and a unity slope. This procedure considers the effects of various types of errors. The presence of random errors in the test method causes points to scatter around the least squares line, and the calculated slope and intercept to slightly depart from unity and zero, respectively. Random errors can be estimated from the standard deviation in the y-direction (also called the “standard deviation of the estimate of y on x”). A proportional systematic error leads to a change in (b), so the difference between (b) and unity provides an estimate of proportional errors. A constant systematic error reflects in a non-zero intercept. If both methods provided identical concentrations in each sample, then the least squares analysis would give a zero intercept and a unity slope. Experimentally, the point of zero intercept and unity slope fell within the joint confidence region for the three analytes. Therefore, the accuracy of the proposed method is not significantly different from that of currently endorsed methods for the same purpose.
Applications. Because no commercially available pharmaceutical dosage forms containing SARA, FLU and OFLO exist, the practical usefulness of the proposed method was determined by using it to analyze two different preparations containing at least one of the compounds. The preparations were spiked with different amounts of the other compounds for analysis. The analytical results, expressed as the average percentage of the nominal content, are summarized in Table 2. The recoveries obtained ranged from 97 to 104%; therefore, the results were quite consistent with the nominal contents and the precision was quite acceptable.
The proposed method was also successfully used to determine SARA, FLU and OFLO in rabbit plasma. For this purpose, reconstituted plasma was spiked with different amounts of the fluoroquinololes. Analyte recoveries were determined by using the standard addition method owing to the strong matrix effects of the plasma. Table 3 shows the assay results, expressed as percent recoveries. As can be seen, all were quite good.
CONCLUSIONS
A method using EEM for the highly sensitive simultaneous determination of overlapping compounds was developed. EEM analysis provides a “fingerprint” consisting of a 3D emission/excitation intensity plot and affords the expeditious, inexpensive analysis of multifluorophore systems, where spectral overlap precludes the use of conventional spectrofluorimetry or even “new” fluorimetric techniques. The problem was solved by application of two different routes using a VASF and an emission scan to ensure complete resolution. The proposed method is simpler than its chromatographic and mass spectrometric counterparts; also, it is less time-consuming than existing LC methods for the simultaneous determination of target fluoroquinolones and requires no internal standard, gradient elution or time programming to adjust excitation and emission wavelengths. The method performs quite well in terms of precision (RSD < 2.5%) and LOD and affords simultaneous quantitation of the three antibiotics in real samples without any pretreatment. All this makes it quite a promising choice for routine analyses.
REFERENCES
Currie, D., Lynas L., Kennedy, D.G., and Mccaughey, W.J., Food Addit. Contam., 1998, vol. 15, p. 651.
Ihrke, P.J., Papich, M.G., and Demanuelle, T.C., Vet. Dermatol., 1999, vol. 10, p. 193.
Ragab Gamal, H. and Amin, A.S., Spectrochim. Acta, Part A, 2004, vol. 60, p. 973.
Golet, A.C., Hartmann Alder, A., Temes, T.A., and Giger, W., Anal. Chem., 2001, vol. 73, p. 3632.
Barrón, D., Jiménez-Lozano, E., Bailac S., and Barbosa, J., Anal. Chim. Acta, 2003, vol. 477, p. 21.
Altiokka, G., Atkosar, Z., and Can, N.O., J. Pharm. Biomed. Anal., 2002, vol. 30, p. 881.
Vílchez, J.L., Ballesteros, O., Taoufiki, J., Sánchez-Palencia, G., and Navalón, A., Anal. Chim. Acta, 2001, vol. 444, p. 279.
Ocaña González, J.A., Callejón Mochón, M., and Barragán de la Rosa, F.J., Talanta, 2000, vol. 52, p. 1149.
Arroyo-Manzanares, N., Huertas-Pérez, J.F., Lombardo-Agüï, M., Gámiz-Gracia, L., and García-Campaña, A.M., Anal. Methods, 2015, vol. 7, p. 253.
Neckel, U., Joukhadar, C., Frossard, M., Jäger, W., Müller, M., and Mayer, B.X., Anal. Chim. Acta, 2002, vol. 463, p. 199.
Pecorelli, I., Galarini, R., Bibi, R., Floridi, Al., Casciarri, E., and Floridi, A., Anal. Chim. Acta, 2003, vol. 483, p. 81.
Bailac, S., Ballesteros, O., Jiménez-Lozano, E., Barrón, D., Sanz-Nebot, V., Navalón, A., Vílchez, J.L., and Barbosa, J., J. Chromatogr. A, 2004, vol. 1029, p. 145.
Lara, F.J., del Olmo-Iruela, M., and García-Campaña, A.M., J. Chromatogr. A, 2013, vol. 1310, p. 91.
D’Agostino, P.A., Hancock, J.R., and Provost, L.R., Rapid Commun. Mass Spectrom., 1995, vol. 9, p. 1038.
Turnipseed, S.B., Roybal, J.E., Pffening, A.P., and Kijak, P.J., Anal. Chim. Acta, 2003, vol. 483, p. 373.
Ballesteros, O., Sanz-Nebot, V., Navalón, A., Vílchez, J.L., and Barbosa, J., Chromatographia, 2004, vol. 59, p. 543.
Ruiz-Viceo, J.A., Rosales-Conrado, N., Guillén-Casla, V., Pérez-Arribas, L.V., León-González, M.E., and Polo-Díez L.M., J. Food Compos. Anal., 2012, vol. 28, p. 99.
Aly, F.A., Al-Tamimi, S.A., and Alwarthan, A.A., Talanta, 2001, vol. 53, p. 885.
Rizk, M., Belal, F., Aly, F.A., and El-Enany, N.M., Talanta, 1998, vol. 46, p. 83.
Ghoneim, M.M., Radi, A., and Beltagi, A.M., J. Pharm. Biomed. Anal., 2001, vol. 25, p. 205.
Xuan, C.S., Wang, Z.Y., and Song, J.L., Anal. Lett., 1998, vol. 31, p. 1185.
Rizk, M., Belal, F., Ibrahim, F., Ahmed, S.M., and Sheribah, Z.A., J. AOAC Int., 2001, vol. 84, p. 368.
Kilic, E., Koseoglu, F., and Akayt, M.A., J. Pharm. Biomed. Anal., 1994, vol. 12, p. 347.
Andrade-Eiroa, A., de Armas, G., Estela, J.M., and Cerdá, V., TrAC, Trends Anal. Chem., 2010, vol. 29, p. 902.
John, P. and Soutar, I., Anal. Chem., 1976, vol. 48, p. 520.
Murillo Pulgarín, J.A. and Alañón Molina, A., Comput. Chem., 1993, vol. 4, p. 341.
Miller, J.N., Analyst, 1984, vol. 109, p. 191.
Murillo Pulgarín, J.A., Alañón Molina, A., and Fernández López, P., Anal. Chim. Acta, 1998, vol. 370, p. 9.
Murillo, J.A., Alañón A., Fernández, P., Muñoz de la Peña, A., and Espinosa, A., Analyst, 1998, vol. 123, p. 1073.
Murillo Pulgarín, J.A., Alañón Molina, A., and Sánchez-Ferrer Robles, I., Appl. Spectrosc., 2010, vol. 64, no. 8, p. 949.
Savitzky, A. and Golay, M.J.E., Anal. Chem., 1964, vol. 36, p. 1626.
Steinier, J., Termonia, Y., and Deltour, J., Anal. Chem., 1972, vol. 44, p. 1906.
Rousseeuw, P.J. and Leroy, A.M., Robust Regression and Outlier Detection, Oxford: Oxford Univ. Press, 1988.
Lark, P.D., Craven, B.R., and Bosworth, R.C.L, The Handling of Chemical Data, Exeter: Pergamon, 1968, ch. 4.
Massart, D.L., Vandeginste, B.G.M., Deming, S.N., and Kaufman, L., in Chemometrics: A Textbook, Vandeginste, B.G.M. and Kaufman, L., Eds., Oxford: Oxford Univ. Press, 1988.
IUPAC, Analytical Chemistry Division. Nomenclature, symbols, units and their usage in spectrochemical analysis. II, Spectrochim. Acta, Part B, 1978, vol. 33, p. 242.
Warren, D. and Crummett, B., Anal. Chem., 1980, vol. 52, p. 2242.
Miller, J.N., Analyst, 1991, vol. 116, p. 3.
Long, G.L. and Winefordner, J.D., Anal. Chem., 1983, vol. 55, p. 712A, 716A, 720A, 722A, 724A.
Clayton, C.A., Hines, J.W., and Elkins, P.D., Anal. Chem., 1987, vol. 59, p. 2506.
Cañadas-Cañadas, F., Espinosa Mansilla, A., and Muñoz de la Peña, A., J. Sep. Sci., 2007, vol. 30, p. 1242.
Funding
The authors gratefully acknowledge financial support from the “Consejería de Educación, Cultura y Deportes de la Junta de Comunidades de Castilla-La Mancha y el Fondo Europeo de Desarrollo Regional” (PEII-2014-008-A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Pulgarín, J.A., Molina, A.A. & Boras, N. Use of Total Fluorescence Spectroscopy for the Highly Sensitive Simultaneous Determination of Fluoroquinolones in Rabbit Plasma. J Anal Chem 76, 733–741 (2021). https://doi.org/10.1134/S1061934821060101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934821060101