Abstract
The compatibility of tetraethoxysilane (TEOS) with polyacrylonitrile (PAN) solutions in dimethyl sulfoxide, the morphology of the mixed systems, and their rheological behavior have been analyzed. The combination of interferometry, refractometry, and optical microscopy has been employed to study the phase equilibrium realized in mixtures of TEOS with dimethyl sulfoxide and a PAN solution and to plot phase diagrams, which have indicated that TEOS is soluble in the PAN solution up to TEOS concentrations of 10–11%. As the TEOS content is increased within a concentration range of 10–20%, an emulsion with droplet sizes up to 40 µm is formed. At higher TEOS concentrations, the macroscopic separation of the system takes place. It has been shown with the use of rotational rheometry that, in the range of solutions, the presence of TEOS leads to a decrease in their viscosity and elasticity, while the viscosity increases in the range of emulsification. The analysis of the dynamic data has, for the first time, shown the bifurcation of the dependences of the storage modulus on the loss modulus at the point of phase separation. The calculation of the characteristic relaxation time of the ternary system and the dependence of this time on TEOS concentration has indicated a dramatic growth of the relaxation time in the region of emulsification, with this growth being determined by the relaxation properties of interfaces. Considering the obtained systems as a raw material for producing composite fibers, a solution droplet has been used to simulate the effect of TEOS on the kinetics of coagulation of PAN solutions with precipitants having different activities. It has been shown that, depending on TEOS concentration, the precipitation may yield gels with the content of this organosilicon additive either uniformly or nonuniformly distributed over the hypothetic cross section of a fiber.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The incorporation of silicon-containing additives into spinning solutions for producing diverse fibers is urgent primarily in the case of precursors for carbon-silicon-carbide fibers. The properties of final fibers equally depend on the structure of a polymer, character of additive distribution in it, interaction between a basic polymer and an additive at different stages of producing hybrid fibers, and conditions of their carbonization [1–3]. An incorporated additive may substantially affect the set of the properties of both a spinning solution and a resulting fiber [4].
The production of hybrid carbon-silicon-carbide fibers has been considered in a number of works, where different methods were used to incorporate silicon-containing additives. For example, the authors of [5] described the incorporation of incompatible silicon-containing compounds (tetraethoxysilane (TEOS), vinyltetraethoxysilane, etc.) into a spinning solution of cellulose with the formation of composite fibers—those being precursors of carbon fibers. This modification of the spinning solution substantially changed the structure of the fibers. Carbonization of cellulose-based composite fibers with silicon-containing additives is characterized by an increased yield of carbon in a final product and the formation of silicon-carbide inclusions [6]. Thus, the presence of an organosilicon additive in a cellulose-based precursor fiber makes it possible to catalyze cellulose pyrolysis and functionalize a final carbon material with silicon carbide nanoparticles.
The incorporation of additives inevitably affects the morphology and rheological properties of systems, thereby directly influencing the parameters of the spinning process and the possibility of fiber formation as a whole. Numerous publications have been devoted to the effects of soluble and insoluble additives on the properties of polyacrylonitrile (PAN) spinning solutions. At the qualitative level, three types of possible compositions may be distinguished: solutions containing additives with viscosities lower and higher than the viscosity of a polymer solution, emulsions with different morphologies, and suspensions of particles with different shapes and sizes.
The preparation of SiC particles from silicon-containing compounds, such as TEOS and methyltriethoxysilane, in the presence of PAN via the sol–gel technology has been described in [7], where PAN was used as a source of carbon at the stage of silicon reduction. In this case, the sol–gel process is represented by the hydrolytic polycondensation of TEOS in a PAN solution followed by its transformation into an oligosiloxane gel, i.e., into a colloidal system that consists of a liquid dispersion medium included into a spatial network formed by dispersed phase particles. By subjecting this gel-like system to thermal treatment, the authors of [7] obtained samples of silicon carbide particles with different densities and porosities. It has been shown that, in the case of the sol–gel process, silicon carbide is formed at a lower temperature to compare with the common process of silicon carbide production via its reduction from silicon oxide in excess carbon.
From the point of view of the goals of this work, the most similar process is the incorporation of a sol, which has been obtained from TEOS in a tetrahydrofuran-containing aqueous HCl solution, into a PAN solution with the formation of optically transparent organoinorganic materials with the fraction of SiO2 formed as a result of TEOS hydrolytic polycondensation equal to 12–93% [8]. SiO2 reduction during the thermolysis yields silicon carbide. The chemical transformations occurring in the course of the preparation of such materials are schematically represented in Fig. 1.
Schematic representation of TEOS hydrolytic polycondensation followed by SiO2 reduction to SiC [5].
The authors of [8] have revealed that, when TEOS is incorporated by the sol–gel method, PAN chains are covalently bonded to the network of SiO2 particles and are uniformly distributed in the system in the entire concentration range of the components due to the precipitation of SiO2 particles being formed from the initially homogeneous system.
Another method for the incorporation of silicon into the carbon matrix consists in the synthesis of PAN in the medium of a sol obtained from a TEOS emulsion in dimethyl sulfoxide (DMSO). This method was compared with the direct incorporation of TEOS in [9]. It was shown that the mechanical mixing of a PAN solution with TEOS upon the addition of stoichiometric amounts of water and HCl for the formation of SiO2 yielded loose and defective fibers. At the same time, the synthesis of PAN in the presence of a sol of the products of TEOS hydrolytic condensation gave rise to the formation of mixed oligosiloxane–polyacrylonitrile sol, from which fibers with no visible defects and signs of phase separation were subsequently obtained.
The authors of the cited publications often consider sols obtained from TEOS in different polar liquids. Therewith, it will be of interest to consider the structure and properties of solutions and emulsions of TEOS in aprotic solvents and in solutions of polymers in such solvents. It is of special interest to study the rheology of emulsions of two low-molecular-weight liquids and more complex systems, in which one of the liquids is a polymer solution. The main factors that affect the rheological properties of emulsions have been considered in recently published monograph [10]. In terms of viscosity, they are, of course, the viscosities of a dispersion medium and a dispersed phase, while the role of an interfacial layer is described in terms of interfacial tension. As for the elastic and relaxation properties, the rheology of an interfacial layer becomes decisive and is often supplemented by allowance for the Laplace pressure.
It is obvious that, when a dispersed phase has a viscosity higher than that of a dispersion medium, droplets remain almost undeformed in a flow; however, if the dispersion medium viscosity becomes rather high, the spherical droplets in the flow are transformed into ellipsoids. The presence of emulsifiers markedly increases interfacial layer elasticity, which is separately introduced into the rheological equation of state [11, 12]. As a whole, the viscoelasticity of emulsions is governed by the deformability of the interfacial layer, while the relaxation (restoration of droplet shape) is determined by the interfacial tension.
However, the situation in which a polymer solution possessing its own viscoelasticity serves as a dispersion medium is seldom considered in the available literature. In this work, we intend to determine the boundaries of TEOS compatibility with PAN solutions in DMSO, study its effect on the rheological behavior of the TEOS–PAN–DMSO three-component systems, and analyze the coagulation of such composite solutions under the action of a precipitant (nonsolvent) for the polymer as the main stage of the wet/dry–wet formation of fibers and films with the aim to eliminate this shortage.
EXPERIMENTAL
Materials
As an object for the study, we selected polyacrylonitrile-based commercial copolymer AN316020 (Good Fellow, United Kingdom) with a weight-average molecular weight of 85 kg/mol, a polydispersity index of 2.1, and the following composition: acrylonitrile, 93.9%; methyl acrylate, 5.8%; and methyl sulfonate, 0.3%. DMSO and TEOS (both of reagent grade, Ekos-1, Russia) were used as a solvent and an additive, respectively. A binary DMSO/distilled water solution (85/15, wt/wt) was prepared to simulate the precipitation process.
To decrease the errors associated with the process of obtaining the systems to be studied, initially, a 20% stock PAN solution in DMSO was prepared (at 50°C and continuous stirring for 20 h). This solution was used to obtain a number of mixed systems with TEOS contents of 0, 0.15, 0.3, 0.45, 0.6, 0.75, 0.8, 1, 2, 5, 10, 15, 20, 25, and 30 wt %. The systems were stirred with a paddle stirrer in sealed containers equipped with Teflon seals for 1 h at 50°С. The temperature of mixing was selected in a manner such that efficient stirring was provided, while hydrolysis of TEOS under the action of small amounts of residual water present in DMSO was prevented. The compositions prepared in this manner were the main objects for the study.
Methods
Morphological study of obtained systems. The morphology of the obtained solutions was studied using a Biomed 6 PO polarization optical microscope (Russia). The procedure was as follows: a droplet (d ≈ 1 mm) of a studied solution was placed onto a microscope slide; then, it was immediately covered with a cover glass (to prevent solvent evaporation and uptake of moisture from air). Preliminarily, a film was glued to the edges of the cover and microscope glasses, to provide a constant gap of ≈100 µm between them. The experiments were performed at a temperature of 25°С.
Determination of the mutual solubility of the components. The compatibility of TEOS with DMSO and PAN solutions was studied by refractometry and microinterferometry. In the first case, a Carl Zeiss Jena 1 refractometer (Germany) equipped with a VPZh-TS-01 liquid thermostat (Russia) capable of maintaining a constant temperature within a range of 20–70°C with an error of 0.1°C was used. To determine the solubility limits in the TEOS–DMSO system, a mixture was prepared with a component ratio of 1 : 1. Below the critical temperature, the mixture was separated into two solutions (TEOS in DMSO and DMSO in TEOS) with different concentrations, with the separation being evident from the presence of an interface. At the critical temperature, the mixture reached the cloud point with the subsequent formation of a true solution.
To plot the phase diagram, the refractive indices of the initial components were preliminarily determined at different temperatures with a step of 10°C and the refractive indices of solutions with preset concentrations of TEOS with a step of 20% at a temperature higher than the solution temperature (47°C). It has been found that, in the region of compatibility, the refractive index linearly depends on the concentration. In the region of incompatibility, the experiment was performed according to the following scheme: a mixture was stirred at a preset temperature for 1 min. After it was separated into two macrophases, a 100-µL sample was taken with a pipette preheated to the experiment temperature, and the refractive indices of the upper and lower phases were determined. Then, the temperature was elevated by 10°C and the experiment was repeated.
In view of the high viscosity of PAN solutions, the above-described procedure appeared to be inapplicable to them. Therefore, to determine the phase compositions of these systems, several PAN solutions were prepared with different TEOS concentrations under continuous stirring, and their refractive indices were determined at different temperatures, while exposing the solutions at each temperature for 20 min. Figure 2 shows the dependence of the refractive index on TEOS concentration for the PAN–DMSO–TEOS system.
The limiting concentration, above which TEOS begins to form a separate phase, was determined from the inflection point. At the same time, the refractive index of the “matrix” (solution of PAN and TEOS in DMSO) becomes constant. In other words, TEOS dissolves in the PAN solution in amounts up to 10–11.5%; then, the phase separation occurs. Thus, the refractometric measurements enable us to determine the boundary between homogeneous mixed solutions of PAN and TEOS in DMSO and heterophase systems.
The phase transition in the aforementioned ternary system was studied in greater detail by interferometry, which was used to solve two problems: the study of TEOS solubility in solutions of PAN in DMSO and the simulation of the interaction of the solutions with a precipitant, the composition of which was selected as was described in [2]. When monochromatic light is passed through a layer, which has a variable thickness and consists of two components brought into a butt contact with one another, interference fringes arise in each component with a step inversely proportional to the refractive index of a component. In the presence of the diffusion interaction, the experiment shows evolution of the position of the interface and the shape of the fringes in the zone of the interdiffusion of the components. The visualization of this evolution makes it possible to assess the compatibility of the components and the rate of the diffusion interaction. In this work, the interference patterns were measured and analyzed by the standard procedure described in [13].
Preliminarily, we studied the interaction of a PAN film with TEOS. For this purpose, a film with a thickness of 117 µm was prepared by casting from a DMSO solution. The film was clamped between glasses, thereby presetting a necessary wedge-shaped gap in the cell, and exposed at 100°C for 5 h until the internal stress relaxation was completed; then the film was brought in contact with TEOS in a “side-by-side” manner. It has been shown that, during a 2-h contact at temperatures of 25–110°C, the interface remains preserved and the interference fringes attributed to PAN and TEOS are not bent. This indicates the absence of the interaction between the components.
The interaction of a PAN solution in DMSO with TEOS is accompanied by the evolution of the interference patterns. These experiments have enabled us to assess the interdiffusion kinetics in the ternary systems. The experiments were performed at temperatures from 25 to 70°C with a step of 10°C.
Rheology. The rheological behavior of the solutions was studied in the continuous and oscillating regimes of the shear deformation with Anton Paar MCR 301 and Thermo Haake RheoStress RS600 rotational rheometers at 25°C. Two different working units were used: coaxial cylinders with an internal cylinder diameter of 10 mm and an intercylinder gap of 0.42 mm and a cone–plane system with a diameter of 60 mm and an angle between the cone generator and the plane of 1°. The following data were obtained: flow curves in a shear rate range of 10–3–104 s–1, amplitude dependences of the complex elasticity modulus in a deformation range of 10–3–103% at frequencies of 1 and 80 Hz, and frequency dependences of the storage and loss moduli within the linear range of viscoelasticity at angular frequencies of 10–3–103 s–1.
Simulation of wet casting of fibers and films. The kinetics of the interaction between 20% PAN solutions in DMSO containing different amounts of TEOS and a precipitant (DMSO/water = 85/15) was studied by optical interferometry at 25°C. This method was previously developed and checked using an optical microscope [2]. The use an interferometer with monochromatic light instead of a microscope has made it possible to markedly increase the image distinctness and monitor the features of the interdiffusion process.
RESULTS AND DISCUSSION
Solubility of TEOS in DMSO and Solutions of PAN
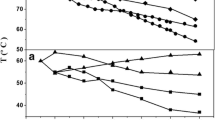
At the first stage of the work, the solubility of TEOS in DMSO was studied by refractometry and the phase diagram presented in Fig. 3 was plotted. It can be seen that the components are completely compatible at temperatures above 47°C, and the diagram refers to the amorphous equilibrium with an upper critical solution temperature. As temperature is decreased, the solution is separated into two phases, with the zone of solubility being shifted toward DMSO.
The addition of the polymer to DMSO, i.e., the use of a PAN solution as a component of the system, substantially deteriorates the compatibility seemingly due to the consumption of free DMSO for PAN solvation. The determination of the boundaries of TEOS solubility in PAN solutions is of importance for the selection of a working concentration range, which provides either a homogeneous or a heterogeneous character of additive distribution in a spinning solution. Let us analyze the situation concerning the dissolution of TEOS in a 20% PAN solution.
Refractometry was used to determine refractive index values, which reflect the concentration of TEOS dissolved in the system. The isotherm of the refractive index (Fig. 2) shows that the maximum concentration of TEOS in a 20% PAN solution is reached when it is incorporated in an amount of 10 wt %; then, TEOS forms a separate phase.
Figure 4 shows the interference patterns that illustrate the final stage of the interaction of a 20% PAN solution in DMSO with TEOS at two temperatures.
The interdiffusion gives rise to concentration gradients of the components, with these gradients causing bending of the fringes near the interface. The fringe shape evolution with time presents information on the redistribution of the components in the diffusion zone, thereby enabling us to calculate the limiting concentration of TEOS in the formed three-component solution. The comparison between the interferometry and refractometry data resulted in the construction of the phase diagrams shown in Fig. 5, in which one component is a PAN solution with a concentration of 10 or 20% and another component is TEOS.
The plotted phase diagrams indicate that, as temperature increases, the solubility of TEOS in PAN solutions remains almost unchanged, while the rise in the concentration of PAN in DMSO from 10 to 20% reduces TEOS solubility in the polymer solution by a factor of nearly 1.5.
The morphology of the DMSO–PAN–TEOS three-component systems was studied by optical microscopy. Figure 6 presents the photographs of bulky samples with different contents of TEOS and the corresponding micrographs of their thin layers. At TEOS contents below 5 wt %, the studied systems show no signs of phase separation; i.e., they are represented by solutions. At a TEOS content of 10%, an emulsion is formed with a particle size of 40 µm. As TEOS concentration further rises to 15–20%, the size and amount of droplets in the emulsion increase. The macroscopic separation of the system begins at an additive content of 20%. At a TEOS concentration of 30%, the system is, in accordance with the phase diagram, separated into two following equilibrium phases: a DMSO solution in TEOS (at the bottom) and a TEOS solution in DMSO (at the top), with the separation being confirmed by refractometric measurements.
Rheology
The main goal of this section is to determine the effect of the addition of partly soluble tetraethoxysilane on the rheological properties of PAN solutions. Figure 7 shows the flow curves for the studied systems in the entire range of compositions.
All studied systems exhibit Newtonian behavior in a wide range of shear rates. At TEOS concentrations higher than 10%, the viscosity begins to decrease with a rise in the shear rate more rapidly than it does for the initial PAN solution. This finding may indicate the effect of the stall or interfacial slip [14]. Therewith, in the region of the non-Newtonian flow, the absolute value of the viscosity varies in a complex manner depending on a system composition. Figure 8 presents the concentration dependence of viscosity at a shear rate of 0.1 s–1. The addition of TEOS in concentrations below 10% leads to a monotonic decrease in the viscosity seemingly due to the dilution of the PAN solution with low-viscosity TEOS. A further increase in the TEOS content leads to a rise in the system viscosity probably due to its separation into two phases, with one phase being enriched with TEOS and the other one with the PAN solution. In addition to the rheological properties of the phases being formed, the deformation of interfaces begins to contribute to the total rheological response.
To determine the region of the linear viscoelasticity, preliminary experiments were performed on the measurement of the complex elasticity modulus and its components (storage modulus G ' and loss modulus G '') at different deformation amplitudes and frequencies of 1 and 80 Hz. For all systems, the real and imaginary components of the complex elasticity were shown to remain unchanged up to a deformation of nearly 30%; therefore, when determining the frequency dependences of the modulus, a deformation regime was selected with an amplitude of 10%, i.e., a fortiori in the region of linear viscoelasticity.
Figure 9 shows the frequency dependences of the storage and loss moduli measured for some ternary systems in the region of their viscoelastic behavior.
The addition of TEOS (15%) decreases the components of the complex modulus, with loss modulus G '' always remaining higher than storage modulus G ' and the system being flowable. The attention is drawn by the following features of these data. First, in the final region, both dependences are described by traditional power functions: the loss modulus is proportional to the frequency, while the storage modulus obeys a power function with an exponent of 1.8–2.0. Second, the character of the concentration dependences of the moduli is, as a whole, consistent with the concentration dependence of viscosity presented in Fig. 7 and corresponds to the transition from solutions to emulsions. However, in the case of low-amplitude harmonic oscillations, the scale of variations in the moduli in the region of the transition is substantially smaller than that in the case of viscosity measured in the continuous deformation mode. This seems to be associated with interface deformability, which responds in different manners to mechanical actions of different types.
By considering the obtained data in the G ' = f(G '') coordinates (the so-called “Cole–Cole” [15] diagrams presented in Fig. 10), the boundary of the solution–emulsion transition may be determined more distinctly: all homogeneous solutions are described by a single function, while an increase in the TEOS concentration above the point of the phase separation leads to its bifurcation.
Taking into account rather a large deviation of the components of the moduli from the single dependence, especially in the range of low stresses, it was of interest to determine the influence of TEOS on the relaxation properties of the systems. For this purpose, the characteristic relaxation time was calculated from the data of the dynamic tests by the following equation [16]:
where |η*| is the complex viscosity and ω is the angular frequency.
The influence of TEOS on the relaxation time at different frequencies was determined by normalizing the obtained data with the use of the ratio between the relaxation time of a TEOS-containing sample and that of an initial PAN solution at a corresponding frequency, i.e., by determining the value of Δλ.
Figure 11 presents a series of Δλ values calculated for different frequencies as depending on the content of TEOS. At a high deformation frequency, the relaxational response of polymer chains alone is recorded; therefore, the Δλ(c) dependences are almost parallel to the abscissa axis in the entire range of TEOS concentrations. In other words, under the conditions of high deformation rates, the structural network of the polymer withstands the entire load irrespective of the presence of the additive (dissolved or isolated with the formation of a separate phase).
A decrease in applied frequency ω reveals the role of interfaces in the relaxation processes, thereby making it possible to distinguish between two types of the behavior of the studied systems, with these types characterizing the region of solutions, which corresponds to TEOS concentrations lower than 10%, and the region of emulsions. In the first case, the addition of TEOS to a PAN solution somewhat reduces the relaxation time due to dilution of the polymer solution. The passage to the heterophase region is accompanied by the formation of numerous interfaces, the response of which to an external action leads to a drastic increase in the relaxation time of the system. The growth of the Δλ values at a TEOS content of ≈15% indicates that the main contribution to the relaxation properties of the emulsions is made by the interfaces.
Simulation of Precipitation Kinetics in TEOS-Containing Solutions of PAN
The simulation of fiber formation from PAN solutions containing additives of TEOS is of special practical interest, because the structure and properties of fibers and films obtained from a solution by casting into a precipitant (the so-called “wet method”) are, to a high extent, governed by the kinetics of the interdiffusion of a precipitant into a jet/fiber and a solvent from a jet/fiber. As has been shown in [2], the simulation of mass transfer by the example of a solution droplet enables one to assess the precipitation kinetics and morphology of a formed interface, the rigidity of which may, when using a “poor” solvent, promote the formation of defects on a fiber surface (Fig. 12).
Comparison between defects observed in a model experiment and cavities that arise in a fiber formed using a “severe” precipitant [2].
In this work, we used another simulation method, namely, the analysis of interference patterns that result from the interaction of a TEOS-containing solution of PAN with a precipitant (a mixture of 85% DMSO and 15% water).
Figure 13 shows the interference patterns that reflect the diffusion interaction of the solutions with the precipitant in the course of time. The interference patterns presented in the left-hand column have been recorded after a 2-s contact between the solutions and the precipitant. It can be seen that, up to a TEOS content of 10%, the stationary interdiffusion of the solvent and precipitate occurs, which is evident from nearly the same bends of the fringes at both sides of the interface. At the same time, the interface remains preserved; i.e., the solution and the precipitant are compatible only partly. However, beginning from a TEOS concentration of 10% the contact of the solution with the precipitant leads to the phase separation, which is a fundamental difference between this precipitation process and that characteristic of the solution of PAN alone (the upper interference pattern). In the case of solutions with high contents of the additive, the diffusion process may be judged only by the right-hand interference pattern attributed to the precipitant, because the resulting emulsions are turbid. Taking into account the less pronounced bend of the precipitant fringes near the interface, we may speak of a slower separation of the solvent from the heterogeneous system to compare with the one-phase (homogeneous) system. The performed analysis has shown that the same precipitant may be either “mild” or “severe” depending on the composition of a system being cast.
CONCLUSIONS
This work has resulted in determining the limiting concentration for the molecular compatibility of TEOS with a PAN solution, with this concentration corresponding to the transition from the solution to an emulsion. Such a boundary determined by refractometry and interferometry lies in the region of 10% TEOS content. At lower concentrations, a molecular solution is formed and low-viscosity TEOS reduces its viscosity. At higher concentrations, phase separation occurs with the formation of emulsions, the composition of which is, in accordance with the phase diagram, conforming to a mixture of two solutions enriched with either a PAN solution or TEOS. This process is accompanied by an increase in the values of all rheological characteristics. The concentration behavior of the characteristic relaxation time, which has extrema at TEOS contents of 10 and 15%, and the bifurcation of the Cole–Cole diagram are of special interest.
The simulation of the precipitation of the systems based on a TEOS-containing solution of PAN has shown that the presence of the organosilicon component in the heterogeneous system substantially affects the kinetics of the mass transfer by decelerating it in the general case.
The results obtained underpin the technological regimes necessary for the formation of hybrid fibers and films on the basis of PAN containing additives of an organosilicon phase. In particular, this concerns the composition of spinning solutions and spinning baths, the mass transfer kinetics upon the wet casting of fibers, and the morphology of hybrid fibers.
REFERENCES
Gupta, A.K., Paliwal, D.K., and Bajaj, P., J. Macromol. Sci., Rev. Macromol. Chem., 1991, vol. 31, p. 1.
Kulichikhin, V.G., Skvortsov, I.Yu., Mironova, M.I., Ozerin, A.N., Kurkin, T.S., Berkovich, A.K., Frenkin, E.I., and Malkin, A.Ya., Adv. Polym. Technol., 2018, vol. 37, p. 1099.
Morris, E.A., Master Thesis (Univ. of Kentucky, 2011).
Chang, H., Chien, A.-T., Liu, H.C., Wang, P.-H., Newcomb, B.A., and Kumar, S., ACS Biomater. Sci. Eng., 2015, vol. 1, p. 610.
Makarov, I.S., Golova, L.K., Kuznetsova, L.K., Bondarenko, G.N., Skvortsov, I.Yu., Mironova, M.V., and Bermeshev, M.V., Khim. Volokna, 2017, no. 2, p. 24.
Makarov, I.S., Golova, L.K., Bondarenko, G.N., Skvortsov, I.Yu., Berkovich, A.K., Bermeshev, M.V., and Mironova, M.V., Khim. Volokna, 2017, no. 4, p. 3.
Raman, V., Bahl, O.P., and Dhawan, U., J. Mater. Sci., 1995, vol. 30, p. 2686.
Wei, Y., Yang, D., and Tang, L., Macromol. Rapid Commun., 1993, vol. 14, p. 273.
Song, Q.-S., Shi, T.-J., and Ma, H.-H., Polym. Bull. (Berlin), 2008, vol. 61, p. 473.
Derkach, S.R., Reologiya emul’sii (Emulsion Rheology), St. Petersburg: Nauka, 2012.
Danov, K.D., J. Colloid Interface Sci., 2001, vol. 235, p. 144.
Fischer, P. and Erni, P., Curr. Opin. Colloid Interface Sci., 2007, vol. 12, p. 196.
Malkin, A.Ya., Experimental Methods of Polymer Physics (Measurements of Mechanical Properties, Viscosity and Diffusion), New Jersey: Prentice Hall, 1983.
Vinogradov, G.V. and Malkin, A.Ya., Reologiya polimerov (Rheology of Polymers), Moscow: Khimiya, 1977.
Cole, K.S. and Cole, R.H., J. Chem. Phys., 1941, vol. 9, p. 341.
Wissbrun, K.F. and Griffin, A.C., J. Polym. Sci., Part B: Polym. Phys., 1982, vol. 20, p. 1835.
ACKNOWLEDGMENTS
We are grateful to the Russian Science Foundation for financial support of the work, project no. 17-79-30108.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Kirilin
Rights and permissions
About this article
Cite this article
Skvortsov, I.Y., Varfolomeeva, L.A. & Kulichikhin, V.G. The Effect of Tetraethoxysilane on the Phase State, Rheological Properties, and Coagulation Features of Polyacrylonitrile Solutions. Colloid J 81, 165–175 (2019). https://doi.org/10.1134/S1061933X19020145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X19020145