Abstract
Arabidopsis thaliana Heynh. (L.) plants of the Columbia ecotype (Col-0) and its ethylene-insensitive etr1-1 (ethylene resistant 1) and ein2-1 (ethylene insensitive 2) mutants were studied. The plants were compared in respect to their cold tolerance conferred by cold acclimation. The tolerance to negative temperature increased in all the three genotypes after 5-day cooling of the plants at 2°C. Meanwhile, a quantitative difference was observed between them: the Col-0 plants survived better than the mutants, and the electrolyte leakage from the tissues reached 50% at higher (by 1°C and more) temperature in the hardened mutants than in the Col-0. Only the mutants manifested the increased lipid peroxidation over the hardening period; this indicates ROS production and general oxidative stress. From this point, we speculated that the tested Arabidopsis genotypes are different in the efficiency of their antioxidant systems. For testing, the total activities of the chief antioxidant enzymes superoxide dismutase (SOD) and catalase, including their isoenzymes, were monitored in the course of hardening. The total SOD activity was found to be lower in the mutants than in the Col-0 both at the normal growing temperature and over the whole time of the cold hardening. The isozyme analysis revealed the link of the reduced total SOD activity of the mutants with the reduced activity of their Cu/Zn-SOD isoforms at 22°C and at the beginning of the hardening. This relationship was supported by the lower relative content of the CSD1 and CSD2 gene transcripts of Cu/Zn-SOD in the mutants in comparison with the Col-0. The decrease in the total catalase activity was also observed and would be ascribed to the lowered activity of the CAT2 isoform. The reported results evidence to the principal feasibility of the ethylene signaling control of the Cu/Zn-SOD and catalase activities associated with the cold acclimation of Arabidopsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The story into the investigation of mechanisms of plant tolerance to low temperatures has covered several decades [1]. This is an important basic problem of plant physiology, which is closely related to agricultural crop production, since low temperatures, frosts, and light frosts reduce the yield. Convincing success has been achieved at present in the study of physiological, biochemical [2, 3], and molecular [4, 5] fundamentals of plant tolerance and adaptations to hypothermia. However, some processes of acclimation and their control are not comprehended enough so far in spite of a great deal of experimental facts [5, 6]. Arabidopsis thaliana is a convenient model organism to study molecular-biological, genetical, and physiological aspects of cold tolerance and, in fact, is one of plants that are best-studied in this regard [6]. Although the establishment of plant tolerance depends on numerous processes occurring over cold hardening, growth suppression is one of the definitive factors determining the course of acclimation and the resultant level of tolerance to hypothermia [3, 4, 7]. As a consequence, many studies have focused on the roles of phytohormones in acclimation processes. Many pertinent publications considered gibberellins, auxins, abscisic acid, and even brassinosteroids [5, 8], but the role of ethylene remains the least studied at this point [8]. Meanwhile, one publication reported an increased ethylene emission under cooling [9]. The authors assumed a direct relation between the ethylene biosynthesis and the degree of the constitutive and the hardening-conferred tolerance. Our work [10] showed that the etr1-1 (ethylene resistant 1) and ein2-1 (ethylene insensitive 2) Arabidopsis mutants possessed less constitutive cold tolerance than the Col-0 ecotype.
A work was published in 2012 that links the ethylene signaling with the well-known C-repeat binding factor (CBF) pathway controlling tolerance to several kinds of stress, including chilling [11]. In the next paper [12], the authors demonstrated the rise in expression of the key protective genes in the ethylene-insensitive etr1-1 and ein2-1 mutants of Arabidopsis and came to a conclusion on the negative influence of the ethylene signal on the tolerance. Among these genes, the study mentioned those of superoxide dismutase (SOD) and catalase—the enzymes affording protection from oxidative stress resulting from various unfavorable factors, including hypothermia. According to the suggestion made, the repression of the protective genes and, consequently, the negative influence of ethylene on the tolerance are eliminated in the Arabidopsis mutants.
In this study, the cold tolerance of Arabidopsis was directly estimated by its exposure to weak freezing (‒1°C), which, as a rule, did not cause damage and ice formation in this plant. In addition, Shi et al. [12] measured the membrane damage by the electrolyte leakage from leaves, though the plants were affected by the ethylene precursor 1-aminocyclopropan-1-carbonic acid rather than hypothermia. Therefore, the cited works [11, 12] do not contain a parallel confirmation of the difference between the genotypes in their acquired tolerance. Instead, the authors visually evaluated the survival and assessed the ethylene effect by the electrolyte evolution that does not appear to be quite correct for the final conclusions. Other authors [13] doubted this inference since, in the transgenic plants of Arabidopsis and grapevine, ethylene up-regulates the cold tolerance by overexpression of the VaERF057 gene encoding transcription of the ethylene response factor (ERF), which is the main participant in the ethylene signaling pathway. After chilling of the ERF-overexpressing transgenic plants, they contained lower amounts of MDA and higher activities of SOD, peroxidase, and catalase than the wild-type. Besides, in the transgenic line highly expressing VaERF05, the transcription of 32 stress-related genes, including CBF1-3, is also intensified. By contrast, application of aminoethoxivinilglicine (the ethylene biosynthesis inhibitor) reduced the cold tolerance of grapevine plants. Therefore, the current literature comprises controversial publications reporting either the decreased [10–12] or increased [13] tolerance of the ethylene-insensitive mutants in comparison with the Col-0. In general, the number of complex studies analyzing the ethylene involvement in the plant cold acclimation is inadequate.
Since the expression of the SOD genes changes after mutation and cold hardening [11], we suggested that the sensitivity to ethylene may affect functioning of the key components (mainly enzymatic) of the plant antioxidant system upon cold acclimation. To test this hypothesis, the total activities of SOD and catalase (and their isoenzymes), in parallel with the cold tolerance, were monitored by different methods in the course of hardening. The objective of the work was to study the role of ethylene in establishing tolerance to hypothermia occurring upon low-temperature hardening, particularly in relation to changes in the activities of the chief antioxidant enzymes. The Arabidopsis genotypes sensitive and insensitive to this hormone were compared in the study.
MATERIALS AND METHODS
Plant material. Plants of Arabidopsis thaliana Heynh. (L.) ecotype Columbia (Col-0) and its ethylene-insensitive etr1-1 and ein2-1 mutants were studied. The seeds were purchased from the Nottingham Arabidopsis Stock Center (Great Britain). The mutants of this species with altered sensitivity to ethylene are known to be convenient objects for investigation of the regulatory role of this hormone in the development of cold tolerance in the course of hardening. After a treatment with ethylene, etiolated seedlings specifically display a so-called triple response. This is a retardation in elongation of the root and hypocotyl together with a radial swelling of the hypocotyl and formation of the apical hook. This response is specific and easily detectable. Owing to it, the ethylene-insensitive mutants in the ETR1 [14], CTR1, and EIN2 genes, encoding, respectively, the ethylene receptor and the components of its signaling pathway, were isolated and genetically characterized. In the etr1-1 mutant, one of the receptors (ETR1) is altered; this leads to the 80% suppression of the ethylene binding and, as a consequence, the full insensitivity to ethylene [14]. The ein2-1 mutant contains one damaged protein of the signaling cascade (EIN2), which is responsible for the signal transduction to the ethylene-regulated transcription factors ERF [15]. The etr1-1 mutant hardly differs from the wild-type plants, while the ein2-1 is notable for the suppressed growth. It is shown that ethylene present in the plant is perceived by the ETR receptor followed by the signal transduction to the genome through the cascade of the intermediate proteins CTR, EIN, and EIL [15].
The plants were grown on a soil mixture based on Agrobalt-S high-moor peat (Rostorfinvest, Russia) at 22°C and under 8-h daily illumination at 100 µmol/(m2 s) in a growth chamber of the phytotron of the Timiryazev Institute of Plant Physiology. The 8-h photoperiod was chosen, because Arabido-psis is a facultatively long-day plant. However, at a shorter day, it reaches the rosette biomass necessary for experiments without passage to the flowering phase. This is especially significant for the tolerance establishment, since flowering plants are poorly hardenable. For estimating the tolerance, the 7–8-week-old plants were used; their leaves of the 5–6 levels were harvested for biochemical analyzes.
Hardening lasted from one to five days at 2°C, under 50 µmol/(m2 s) illumination, and 16-h photoperiod in the climatic chamber KWB-240 (Binder GmbH, Germany). The appropriate temperature and duration of the procedure were chosen in the preliminary experiments [16]. The control was represented by the plants that were not subjected to the hardening procedure.
Plant cold tolerance was evaluated before and after the hardening. The whole plants were placed in the growth chamber at 0°C, and the temperature was lowered down to –3°C throughout one hour, and the plants were kept for one day under these conditions. This allowed plants to undergo the second hardening phase to fulfill the frost resistance pertinent to frost-tolerant species. Afterwards, the temperature was sequentially decreased by 1°C to –4°, –5°, –6°, –7°, and –8° within 1 h, and the plants were left at every negative temperature for 1 day. After the freezing at the indicated temperatures, the plants were allowed to thaw for 1 day at 4°C. Their survival was estimated after 3-day maintenance at 22°C under light. In addition to the survival, plant cold tolerance was evaluated by the Hepburn’s conventional method based on measurement of electrolyte leakage from the cells. This index was derived as a percentage of the maximal value of conductivity measured after the plant boiling with the following formulae:
where H is tolerance; EL is the maximal leakage of electrolytes; and Еinit, Ecool, and Еboil represent the electrolyte leakage before the sample cooling, after cooling, and after boiling, respectively.
Rate of oxidative processes was estimated by the content of MDA—one of lipid peroxidation final products. This index was determined with the assay based on the reaction with thiobarbituric acid [16] and was expressed as a percentage of the initial value obtained before the hardening.
Soluble proteins were isolated from the leaves with a buffer solution containing 50 mM Tris–HCl (рН 7.6), 3 mM EDTA, 250 mM sucrose, 3.6 mM cysteine, 5 mM ascorbic acid, 3 mM MgCl2, 2 mM DTT, and 2 mM PMSF [17]. The enzymatic activities were assayed in the extract after its desalination in PD-10 midiTrap G-25 columns (GE Healthcare, United States).
SOD activity was assayed in the system wherein superoxide anion radicals are generated in the riboflavin photooxidation and are scavenged by nitroblue tetrazolium (NBT) [17]. One unit of the SOD activity was defined as an amount of the enzyme inhibiting 50% the formazan yield in the reaction with NBT. The activity was expressed as units/g fr wt.
Isoenzyme content of SOD was analyzed with electrophoresis under nondenaturing conditions in 15% separating PAAG gel (1 mm as thick) in a Mini PROTEAN Tetra device (Bio-Rad, United States) by the conventional Ornstain-Davis technique. To each well, we added 20–40 µg protein plus 225 mg/mL sucrose (to make the samples heavier) and 2 µL/mL of 0.5% bromphenol blue. Electrophoresis was carried out for 2 h at 4°C and 180 V using a PowerPac™ Universal Power Supply (Bio-Rad). To verify the effectiveness of an alignment of protein samples, one of the gels was stained for 1 h with 0.1% Coomassie G-250. Before staining, the sample was fixed for 30 min in the mixture of 10% (NH4)2SO4, 3% H3PO4, and 20% ethanol according to the standard procedure. The protein content in the sample was assayed with the bicinhoninic method.
To visualize activities of SOD isozymes, the gels were incubated in the dark for 30 min in the mixture of 30 µM riboflavin and 245 µM NBT in 50 mM Tris-HCl, pH 7.6. The gels were rinsed with buffer solution and placed under high light where they turned violet. The gel areas containing SOD activity remained colorless [18]. The visually estimated degree of the background discoloration showed the activities of SOD isozymes.
To identify the types of SOD isozymes, an inhibitory analysis was employed. For this purpose, the gels were alternatively kept in 3 mM KCN or 5 mM hydrogen peroxide for 30 min [18] followed by the staining described above.
Catalase activity was determined through the rate of H2O2 decomposition [16] and was expressed as mmol H2O2/(g protein min).
Changes in activity of catalase isozymes were estimated with electrophoresis under nondenaturing conditions in 10% PAAG (gel thickness 0.75 mm) for 20 h at 4°C and 80 V. Each sample contained 7 µg protein. The gels were stained by the method based on reduction of potassium ferricyanide (III) K3(Fe(CN)6) by hydrogen peroxide to potassium ferrocyanide (II) K4(Fe(CN)6), which reacts with iron chloride (III) FeCl3 yielding the colored product berlin blue [19]. The color was absent in catalase-containing loci since the enzyme decomposes H2O2. To visualize activities of catalase isozymes, the gels were incubated in 50 mM Tris-HCl buffer, pH 7.6, containing 4 mM H2O2 for 5 min and then were washed in the H2O2-free buffer. The gels were specifically stained for catalase activity in the solution containing 1% potassium ferricyanide (III) and 1% iron chloride (III) in which they were incubated for 5 min until the appearance of bright bands on a green background. For a quantitative comparison of activities of the isoforms, the gels were scanned, and the image was inverted and analyzed with a ONE-DScan V 1.3 program (Scanalitics, CSP Inc., United States). The intensity of track staining, in comparison with Col-0 inside one gel, displayed the relative activity of catalase.
Total RNA was isolated from the leaves using a Spectrum Plant Total RNA Kit (Sigma, United States) according to the manufacturer’s protocol. The RNA content was evaluated with a ND-1000 spectrophotometer (Nanodrop Inc., United States). The purity of the isolated RNA was estimated by the A260/A280 ratio, which was never below two. The integrity of the RNA preparation was assessed through the PCR analysis.
Reverse transcription was undertaken with an MMLV RT kit (Evrogen, Russia) and the corresponding protocol. The obtained cDNA was employed for real time PCR, which was performed with a CFX96 Touch Real-Time PCR Detection System amplifier (Bio-Rad) with a qPCRmix-HS SYBR reagent kit (Evrogen). The relative content of transcripts for a particular gene in a sample was derived with the ΔΔCT method and was expressed in arbitrary units (the relative transcript level compared to levels before the hardening). The indices of the RT-PCR efficiency, calculated from the calibration curves for all primer pairs, were allowed for the analyses [20]. The primers for the studied Cu/Zn-SOD genes were chosen from the NCBI database and the Primer3Plus internet resource: CSD1 (AT1G08830)—(F) AGATTCCTCTTACTGGACCAAACTC, (R) TGCGTTTCCAGTAGCCAGGC; CSD2 (AT2G28190)—(F) TCCGCGGCGTCTCTCTCAAC, (R) TCGCCGCGGAAACAACTGTC; CSD3 (AT5G18100)—(F) CCACCGAACGAGGAAGAGCG, and (R) ATGCACAACAACCG-CCCTCC. Primers for the reference genes were also selected, namely, the elongation factor 1α eEF1a (AT5G22220)—(F) TCCCATCTTCTAAGCGGCAGC, (R) TCAGCAACAGCTCCAC-CACC, and actin ACT2 (AT3G18780)—(F) AACAGCAGAGCGGGAAATTG, (R) GCAGCTTCCATTCCCACAAA.
Statistics was performed with the SigmaPlot 12.3 program. The initial data were verified for the correspondence to a normal distribution and then were processed with Student’s t-test at P = 0.05. Every experiment included three to six replications with three plants in each. The figures and tables represent means and their SEs.
RESULTS AND DISCUSSION
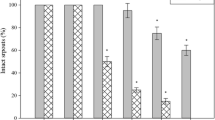
We earlier established that hardening of the Ar-abidopsis ecotype Col-0 elevated the low-temperature tolerance of the plants [16]. For this reason, the present study was primarily focused on the capability of the Arabidopsis ethylene-insensitive mutants of becoming hardened to low temperature. The difference between the mutants and the Col-0 was also planned to be tested in this regard. In our experiments, the plants representing all the three genotypes were hardened and then subjected to frost. It was visually found that the unhardened plants of the two ethylene-insensitive mutants badly suffered after an exposure to –4°C. This was seen as an infiltration, turning the leaves to dark green, and a loss of their turgor. By contrast, all the unhardened Col-0 plants survived (Fig. 1). The photo also demonstrates that the rosettes of the hardened Col-0 plants fully survived the keeping at ‒7°C. The mutants carried damages whose degree was difficult of visual evaluation. Nevertheless, the hardening procedure elevated the cold tolerance of the Col-0 stronger than that of the mutants. Some Col-0 plants survived even after the exposure at –8°C, which was, however, lethal to all the mutant plants.
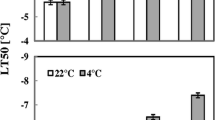
In addition to the visual inspection, the tolerance (H) of the hardened and unhardened plants of all the genotypes was estimated by the electrolyte leakage from plant tissues. This parameter, inversely related to the tolerance, indicates the rate of integrity or, on the contrary, the rate of damage to cellular membranes and even cell death. This makes possible quantitative determination of differences in the resistance of the tested plants. Like the case of the visual estimation, all three genotypes were found to be capable of cold acclimation. However, in the Col-0 plants, the temperature of the 50% electrolyte leakage differed from those in the etr1-1 and ein2-1 mutants by one and two degrees, respectively (Table 1). Consequently, the hardening procedure was less efficient towards the cold tolerance in the ethylene-insensitive Arabidopsis mutants than in the Col-0. In other words, both methods of tolerance evaluation indicated that this trait was weaker in the mutants in comparison with the Col-0 plants.
To support the data on the plant tolerance, the intensity of lipid peroxidation was also evaluated. This process represents not only the tolerance level and the state of cellular membranes but also the relation between ROS generation and antioxidant-mediated inactivation. This determines the level of membrane damage caused by the chilling-originated oxidative stress. It was found that the rate of lipid peroxidation increased in the mutants but did not significantly change in the Col-0 over the hardening (Fig. 2). The rise in this parameter was maximal in the ein2-1 mutant. On day three of the hardening, it almost exceeded 60% its prehardening level and remained this high by day five. In the etr1-1, the MDA content increased by 60% only on day four followed by a slight decrease but it remained higher than in the Col-0.
These data, together with our measurements of the ROS generation [21], suggest the weaker antioxidant system in the mutants than in the Col-0 plants. To test the suggestion, we analyzed dynamics of the activities of the key antioxidant enzymes SOD and catalase in vitro and those of their isoenzymes in situ. First of all, it was necessary to reveal possible differences between the genotypes in the total SOD activity, since this important enzyme actualizes the first-line anti-ROS defense due to inactivation of superoxide anion-radical. If the differences are found, it was planned to assess isozymes responsible for them.
The total SOD activity in vitro was found to be lower in the mutants than in the cold-resistant Col-0 plants both before and during the hardening. Upon plant growing without cooling, this parameter was approximately 125 units/g fr wt in the Col-0, whereas it was three and four times lower in the etr1-1 and ein2-1 mutants, respectively (Fig. 3a). Over the cold acclimation of the Col-0, its total SOD activity significantly doubled and reached the maximum on day three. By day five of the hardening, the activity slightly decreased but remained 50% higher in comparison with the unhardened control. Unlike the Col-0, the total SOD activity of the etr1-1 hardly changed over the hardening and even decreased in the ein2-1 by day five. The data presented imply that the relatively weak cold tolerance of the mutants might be accounted for by the inadequate activity of antioxidant enzymes, especially SOD. Since plants contain three isoform types of this enzyme, the next step of the work was aimed at analyzing the SOD isozymes in respect to their activities in the course of cold acclimation of the three plant genotypes.
Dynamics of total SOD activity (a) in A. thaliana plants of the Col-0 ecotype (1) and its ethylene-insensitive etr1-1 (2) and ein2-1 (3) mutants in the course of 5-day hardening at 2°C. Different letters designate means significantly differing at P ≤ 0.05. (b) Identification of SOD types in vegetating A. thaliana plants of the Col-0 ecotype with inhibitory analysis: (1) without inhibitors; (2) with 5 mM KCN; (3) with 3 mM H2O2; M—markers of molecular weight (band 1 corresponds to Mn-SOD, bands 2–4 correspond to Fe-SOD, and bands 5–7 correspond to Cu/Zn-SOD). (c) Dynamics of activities of SOD isozymes in A. thaliana plants of 12etr1-1 3ein2-1 mutants in the course of 5-day hardening at 2°C.
Literature reports seven Arabidopsis genes encoding seven SOD isozymes that are distinct from each other in the cofactor’s metal and subcellular localization. Of them, the CSD1, CSD2, and CSD3 genes encode Cu/Zn-SOD situated in the cytoplasm, chloroplasts, and peroxisomes, respectively. The FSD1, FSD2, and FSD3 genes encode chloroplastic Fe-SOD and the MSD1 gene codes mitochondrial Mn-SOD [22, 23].
In the plants of the studied genotypes, all three SOD types were identified in gels of native electrophoresis with a use of inhibitory analysis. The entire isozimic spectrum was represented by all the seven isoform known for this plant species. They were identified as one Mn-, three Cu/Zn-, and three Fe-SOD enzymes (Fig. 3b). These results are consistent with those obtained by the analysis of A. thaliana genome sequences [23, 24].
Later on, the contributions of activities of particular isoforms to the total SOD activity were assessed in the Arabidopsis Col-0 ecotype and its ethylene-insensitive mutants in the course of their cold acclimation. For this purpose, the bleaching rates were compared at the gel sites where SOD isozymes were present (Fig. 3c). In the Col-0, the activities of Cu/Zn isoforms were the most changeable and attained the maximum within the first day of the hardening. It appears that the rise in the total SOD activity, accompanying the cold acclimation of these plants, occurred at the expense of only Cu/Zn isoenzymes. In the ethylene-insensitive mutants, the activities of three Cu/Zn-SOD isoforms were low both before and during the whole hardening period. On day five of the hardening, all the three genotypes manifested only two active Cu/Zn-SOD isoforms. The results suggest that the total SOD activity of the mutants was decreased due to the considerable diminishing of the Cu/Zn-SOD component. Such relationships are described in literature. For instance, after a short-term UV-B irradiation of sage plants, some inhibition of total SOD activity occurs over the first 30 h at the expense of a significant reduction in Fe-SOD and Cu/Zn-SOD activities [25].
In Arabidopsis, the SOD gene expression changes as a result of mutations and hardening [11]. We also showed that the Cu/Zn-SOD activity decreased in the etr1-1 and ein2-1 mutants in the course of hardening. Starting from these facts, we evaluated the relative transcript levels for the CSD1, CSD2, and CSD3 genes of Cu/Zn-SOD. It should also be noted that Cu/Zn-SOD is apparently the most important SOD isoform since its expression enhances in the plants subjected to various stressors [22–24]. In our experiments, in the plants before hardening (constitutively, at 22°C), the relative transcript content of all the three Cu/Zn-SOD isoforms was close in the Col-0 and etr1-1, while it was twice as high in ein2-1. After the hardening, the character of the CSD gene expression was similar, with only quantitative differences, in all the tested genotypes. Thus, the cooling enhanced the CSD1 and CSD2 transcription in the Col-0 four times and that in the ein2-1 two times. The CSD3 expression decreased two times in all the genotypes (Table 2). Therefore, our data agree with those of Shi et al. [13] who reported the increased expression of the two Cu/Zn-SOD protective genes. However, the comparison of the total SOD activity and those of its isozymes revealed the decrease in the Cu/Zn-SOD activity over the cold acclimation of these plants. This example demonstrates that evaluation of only transcript levels of protective enzymes is inadequate to judge about plant tolerance; the activity of corresponding enzymes must also be analyzed. The existing inconsistence in activities of SOD isoforms and transcription levels of their genes is intensively discussed in literature. Thus, it is assumed that the activity of the cytosolic Cu/Zn-SOD isoform is determined by the availability of its mRNA, and the activity of the plastidic Fe-SOD is regulated at the substrate (allosteric) level rather than the level of transcript changes in the FSD gene [25]. In addition, expression of the CSD1 and CSD2 in Arabidopsis increases in response to ozone, UV-B, and high light [25]. Therefore, CSD2 participates in plant protection from the photooxidative stress. This conclusion is supported by a phenotypical analysis of the csd2 knockdown mutant, which is revealed to display the lower photosynthetic activity, lower chlorophyll content, and slower growth. It is also found that the activity of the CSD2 protein does not reflect the character of the CSD2 mRNA regulation that implies the existence of translational or posttranslational control level of the CSD2 protein [23, 26]. Under oxidative stress, one of the reported regulatory mechanisms of the CSD1 and CSD2 transcription at the posttranscriptional level is the suppression of the miRNA expression, which is a suppressor of the CSD1 and CSD2 mRNAs [26]. Therefore, regulation of activities of plant Cu/Zn isoforms of SOD was investigated, including the regulation of SOD activity by ethylene upon hardening to negative temperatures. However, because of the absence of a complex analysis performed at different regulatory levels, a comprehensive concept of this phenomenon is hard to elaborate.
The plant cells contain another principal enzyme, catalase, which fulfills one of the key functions in the antioxidant system. Catalase decomposes hydrogen peroxide, including the product of SOD. The reaction is energy-independent unlike those of peroxidases, which require reductants (ascorbate, phenols, etc.). In our experiments, both mutants manifested lower catalase activity than the wild-type plants, namely, 85 and 50% of the Col-0 level in the etr1-1 and ein2-1, respectively (Fig. 4a). In the wild type, the activity decreased to some extent over the first day of hardening and hardly changed during subsequent days. In the etr1-1, the total catalase activity also diminished (by 60% over the third day of the hardening) but it returned to the level of unhardened plants by day five. In the ein2-1, the total catalase activity was the least among the studied genotypes before the hardening. Over the hardening period, it slightly increased and kept to this level for all last time. By day five, the total catalase activity became comparable in the three genotypes.
The relative contributions of catalase isoforms to changes in its total activity (Fig. 4a) was estimated by the degree of gel bleaching caused by the isoforms under normal conditions. As a consequence, the relative activity of the isoenzymes in the dynamics of the cold acclimation of the plants of three genotypes was analyzed in comparison with the Col-0 (Fig. 4b). In A. thaliana, the CAT1 catalase isoform is known to be active in the pollen and seeds, CAT2 in the leaves, and CAT3 in the conducting tissues [27]. These data, together with the results from the analysis of the Ara-bidopsis catalase isoforms with native electrophoresis and immunodetection with antibodies to catalase [18], suggest that the most active isoform found in the Col-0 corresponds to the CAT2 homotetramer of the photosynthetic tissues, and the second isoform of the gel belongs to the CAT3 of the vessels. Figure 4b clearly shows that the catalase activity of the CAT2 isoform is much higher than that of CAT3 and presumably determines the changes in the total enzymatic activity.
In the Col-0 plants, digitization by the ONE-DScan V 1.3 program of the data obtained with the inverted gels revealed that the staining intensity and, consequently, the activity of the CAT3 isoform increased over the hardening period. However, it markedly decreased afterwards and was as small as 20% of the unhardened control by day five. The activity of the CAT2 isozyme remained more than six times higher than that of CAT3 both before and during the hardening. The etr1-1 mutant displayed a similar character of changes in activities of both isoforms upon the hardening. In the ein2-1, in contrast to Col-0 and etr1-1, the CAT3 activity was fivefold higher than that of CAT2 but significantly decreased in comparison with these genotypes in the course of hardening. In the ein2-1, the CAT2 activity was, on the contrary, lower than in the Col-0 and etr1-1 before the hardening but tended to increase although weaker than in two other genotypes over the acclimation.
Therefore, the analysis of the relative activity of catalase isoforms in a gel allows a suggestion that the total activity of this enzyme mainly belongs to the CAT2 isoform in the Col-0 and etr1-1 plants both before and during the hardening; simultaneously, the CAT3 activity significantly decreased. Meanwhile, in the ein2-1 mutant, CAT3 was more active than CAT2 before the cooling but the activity was redistributed between the isoforms over the hardening. As a result, the CAT2 activity of this mutant became comparable with its activity in other genotypes by day five, while the CAT3 activity was close to zero. These data coincided with the total catalase activity on the fifth day of hardening, when the differences between the three genotypes became smoothed.
It should be noted that the total catalase activity, which was measured by a classical method and was expressed in terms of mmol H2O2/min, decreased in the three genotypes by the end of the first hardening day. At the same time, the gel analysis (visual and ONE-DScan-mediated) revealed the simultaneous increase in the enzymatic activity in the Col-0 and etr1-1. The discrepancy might be explained by a peculiarity of the activity assay; when it operates within one gel, it can only assess relative contributions of isoforms into a total activity.
The expression of catalase genes, like those of SOD, is controlled by different pathways [28], for example, by regulation at the transcription level. In general, the regulatory mechanism of catalase gene expression may involve receptors of red and blue light and can respond to contact with hydrogen peroxide, abscisic acid [28], salicylic acid, nitric oxide, hydrogen sulfide, etc. [29]. Another path of catalase activity regulation operates at the posttranscriptional level. One of the well-studied regulatory mechanisms of plants is a decrease in a repeated catalase synthesis under stress conditions [27]. Catalase is a hypersensitive protein with a high turnover rate. Such stressors as cold, salinization, and high light may diminish its activity or reduce the number of protein molecules of catalase due to intensified inactivation or decreased capability of protein substitution. In rye plants, the CAT1 resynthesis proceeds under posttranscriptional control through mRNA methylation, which is stimulated by blue light and peroxides [27].
From literary and our experimental data, we assume that the activities of catalase isoforms may be related to ethylene sensitivity fulfilled through several receptors.
Therefore, we can infer that Arabidopsis, in contrast to chilling-sensitive plants, does not undergo a sharp increase in lipid peroxidation intensity over the low-temperature acclimation. This points to the highly efficient constitutive antioxidant protection, innate in this species. We showed that the ethylene-insensitive mutants were less tolerant to cold than the Col-0 plants, either before or during hardening (Figs. 1, 3, Table 1). Meanwhile, the mutants were also capable of cold acclimation under the same conditions as Col-0 but less effectively, because their critical 50% leakage of electrolytes was attained at the higher temperature. It was also found that the ethylene-insensitive mutants, under control conditions and upon hardening, despite the induction of transcription of the CSD1 and CSD2 genes, possessed the reduced total activities of both SOD (Fig. 3a) and catalase (Fig. 4a). In the case of SOD, this might be accounted for by the decreased activities of Cu/Zn isoforms, especially at the beginning of the hardening (Fig. 3c). The results allow us to suggest that the genes of the Cu/Zn-SOD isoforms are regulated by the ethylene signaling cascade. The total activity of catalase, which is determined by CAT2, was also higher in the Col-0 plants than in the mutants both before and during hardening. Besides, the mutants exhibited a stronger decrease in the total SOD than in the total catalase activity regardless of being before or during the hardening. Here, the difference in the total SOD activity decreased in the mutants to a larger extent than the total catalase activity, at least threefold, while the catalase activity was comparable in the three genotypes on day five. SOD and catalase represent the important enzymatic component of the general antioxidant system. Therefore, the inadequate activity of this component over the time of hardening might entail intensification of lipid peroxidation and decrease in the low-temperature tolerance in the ethylene-insensitive mutants.
REFERENCES
Tumanov, I.I., Fiziologiya zakalivaniya i morozostoikosti rastenii, (Physiology of Hardening and Frost Resistance of Plants), Moscow: Nauka, 1979.
Titov, A.F., Akimova, T.V., Talanova, V.V., and Topchieva, L.V., Ustoichivost’ rastenii v nachal’nyi period deistviya neblagopriyatnykh temperatur (Stability of Plants in the Initial Period of Action of Unfavourable Temperatures), Moscow: Nauka, 2006.
Trunova, T.I., Rastenie i nizkotemperaturnyi stress. 64‑e Timiryazevskoe chtenie (Plant and Low Temperature Stress, the 64th Timiryazev Lecture), Moscow: Nauka, 2007.
Kurbidaeva, A.S. and Novokreshchenova, M.G., Genetic control of plant resistance to cold, Genetika, 2011, vol. 47, no. 3, p. 735.
John, R., Anjum, N.A., Sopory, S.K., Akram, N.A., and Ashraf, M., Some key physiological and molecular processes of cold acclimation, Biol. Plant., 2016, vol. 60, p. 603.
Liu, Y., Dang, P., Liu, L., and He, C., Cold acclimation by the CBF–COR pathway in a changing climate: lessons from Arabidopsis thaliana,Plant Cell Rep., 2019, vol. 38, p. 511. https://doi.org/10.1007/s00299-019-02376-3
Dubois, M., von den Broeck, L., and Inzé, D., The pivotal role of ethylene in plant growth, Trends Plant Sci., 2018, vol. 23, p. 311. https://doi.org/10.1016/j.tplants.2018.01.003
Gamalero, E. and Glick, B.R., Ethylene and abiotic stress tolerance in plants, in Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change, Ahmad, P. and Prasad, M.N.V., Eds., 2012, p. 395. https://doi.org/10.1007/978-1-4614-0815-4_18
Catala, R., Lopez-Coborro, R., Castellano, M.M., Angosto, T., Alonso, J.M., Ecker, J.R., and Salinas, J., The Arabidopsis14-3-3 protein RARE COLD INDU-CIBLE 1A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation, Plant Cell, 2014, vol. 26, p. 3326. https://doi.org/10.1105/tpc.114.127605
Popov, V.N., Deryabin, A.N., Astakhova, N.V., Antipina, O.V., Suvorova, T.A., Alieva, G.P., and Moshkov, I.E., Ethylene-insensitive Arabidopsis mutants etr1-1 and ein2-1 have a decreased freezing tolerance, Dokl. Biochem. Biophys., 2019, vol. 487, no. 1, p. 269.
Shi, Y., Tian, S., Hou, L., Huang, X., Zhang, X., Guo, H., and Yang, S., Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and Type-A ARR genes in Arabidopsis, Plant Cell, 2012, vol. 24, p. 2578. https://doi.org/10.1105/tpc.112.098640
Shi, Y., Ding, Y., and Yang, S., Cold signal transduction and its interplay with phytohormones during cold acclimation, Plant Cell Physiol., 2015, vol. 56, p. 7. https://doi.org/10.1093/pcp/pcu115
Sun, X., Zhao, T., Gan, S., Ren, X., Fang, L., Karungo, S.K., Wang, Y., Chen, L., Li, S., and Xin, H., Ethylene positively regulates cold tolerance in grapevine by modulating the expression of ETHYLENE R-ESPONSE FACTOR 057, Sci. Rep., 2016, vol. 6, p. 1. https://doi.org/10.1038/srep24066
Bleecker, A.B., Estelie, M.A., Somerville, C., and Kende, H., Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana,Science, 1988, vol. 241, p. 1086.
Ju, C. and Chang, C., Mechanistic insights in ethylene perception and signal transduction, Plant Physiol., 2015, vol. 169, p. 85. https://doi.org/10.1104/pp.15.00845
Sin’kevich, M.S., Selivanov, A.A., Antipina, O.V., Kropocheva, E.V., Alieva, G.P., Suvorova, T.A., Astakhova, N.V., Moshkov, I.E., Activities of antioxidant enzymes of Arabidopsis thaliana plants during cold hardening to hypothermia, Russ. J. Plant Physiol., 2016, vol. 63, p. 749. https://doi.org/10.1134/S1021443716060108
Naraikina, N.V., Sin’kevich, M.S., Demin, I.N., Selivanov, A.A., Moshkov, I.E., and Trunova, T.I., Changes in the activity of superoxide dismutase isoforms in the course of low-temperature adaptation in potato plants of wild type and transformed with Δ12‑acyl-lipid desaturase gene, Russ. J. Plant Physiol., 2014, vol. 61, p. 332.
Miszalski, Z., Lesak, I.S., Niewiadomska, E., Baczek-Kwinta, R., Lüttge, U., and Ratajczak, R., Subcellular localization and stress responses of superoxide dismutase isoforms from leaves in the C3CAM intermediate halophyte Mesembryanthemum crystallinum L., Plant Cell Environ., 1998, vol. 21, p. 169.
Zimmermann, P., Heinlein, Ch., Orendi, G., Zentgraf, U., Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh, Plant Cell Environ., 2006, vol. 29, p. 1049.
Rao, X., Huang, X., Zhou, Z., and Lin, X., An improvement of the ΔΔCT method for quantitative real-time polymerase chain reaction data analysis, Biostat. Bioinforma. Biomath., 2013, vol. 3, p. 71.
Sin'kevich, M.S., Antioxidant system for Arabidopsis plants during cold hardening, Mater. Nauch. konf. s mezhd. uchastiem i shkoly molodykh uchenykh “Signal’nye sistemy rastenii: ot retseptora do otvetnoi reaktsii organizma” (Proc. Conf. with Int. Participation and School for Young Scientists “Signal Systems of Plants: From Receptor to the Response of the Organism”), 2016, p. 250.
Saibi, W. and Brini, F., Superoxide dismutase (SOD) and abiotic stress tolerance in plants: An overview, in SuperoxideDismutase, Magliozzi, S., Ed., Nova Sci. Publ., Inc., 2018, p. 101.
Kliebenstein, D.J., Monde, R.-A., and Last, R.L., S-uperoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization, Plant Physiol., 1998, vol. 118, p. 637. https://doi.org/10.1104/pp.118.2.637
Alscher, R.G., Ertuk, N., and Heath, L.S., Role of superoxide dismutases (SODs) in controlling oxidative stress in plants, J. Exp. Bot., 2002, vol. 53, p. 1331.
Radyukina, N.L., Shashukova, A.V., Makarova, S.S., and Kuznetsov, Vl.V., Exogenous proline modifies differential expression of superoxide dismutase genes in UV-B-irradiated Salvia officinalis plants, Russ. J. Plant Physiol., 2011, vol. 58, p. 51.
Sunkar, R., Kapoor, A., and Zhu, J.K., Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by down-regulation of miR398 and important for oxidative stress tolerance, Plant Cell, 2006, vol. 18, p. 2051. https://doi.org/10.1105/tpc.106.041673
Mhamdi, A., Queval, G., Chaouch, S., Vanderauwera, S, van Breusegem, F., and Noctor, G., Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models, J. Exp. Bot., 2010, vol. 61, p. 4197. https://doi.org/10.1093/jxb/erq282
Yang, T. and Poovaiah, B.W., Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin, Proc. Natl. Acad. Sci. U. S. A., 2002, vol. 99, p. 4097. https://doi.org/10.1073/pnas.052564899
Kolupaev, Yu.E., Karpets, Yu.V., and Kabashnikova, L.F., Antioxidative system of plants: cellular compartmentalization, protective and signaling functions, mechanisms of regulation (review), Appl. Biochem. Microbiol., 2019, vol. 55, p. 441.
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation and was carried out through State Task no. AAAA-A19-119080690056-3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any work conducted on animal or human participants.
Additional information
Translated by A. Aver’yanov
Abbreviations: CAT1, CAT2, CAT3—isoforms of catalase; CBF—C-repeat binding factor; ein2-1—ethylene insensitive 2 mutant; ERF—ethylene response factor; etr1-1—ethylene resistant 1 mutant; NBT—nitroblue tetrazolium; SOD—superoxide dismutase.
Rights and permissions
About this article
Cite this article
Sin’kevich, M.S., Naraikina, N.V., Alieva, G.P. et al. The Difference in Low-Temperature Tolerance of Arabidopsis thaliana Plants and Its Ethylene-Insensitive Mutants Is Related to Activities of Antioxidant Enzymes. Russ J Plant Physiol 67, 1083–1093 (2020). https://doi.org/10.1134/S1021443720050155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443720050155