Abstract
The idea of sustainable and circular utilization of difficult to decompose plastics is pursued to add value to other products. New epoxy mortars suitable for construction repair made from epoxy oligomer, amine and modified PET waste hardeners mixed with sand were prepared. Three types of carboxyl terminated PET (CTPET) were used as co-hardeners, along with an amine, to prepare new combination linkage epoxy mortars. Investigation of the viscoelastic properties revealed that the CTPETs influenced the storage modulus at glassy state and the rubbery plateau, indicative of interfacial adhesion between the epoxy matrix and sand aggregates. Additionally, the damping behavior and glassy temperature were increased with the addition of CTPET. Moreover, the combination linkage of ester groups from the CTPET likely contribute to the enhancement of compressive strength and flexural strength in the epoxy mortar. The results of absorption and flexural change after immersion in solution, as well as the interfacial flexural bonding strength with ordinary cement-based material were also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Mortar is a building material that can be used in many applications, such as plastering coatings, grouts, sealants, and repair materials. In addition to the general mortar, nowadays mortars with special ingredients, known as modified mortars, are available. These special mortars are mixed with polymers to improve some of their properties. For example, elastomers can be added to provide a more flexible mortar. On the other hand, mixing with an epoxy resin results in a stronger mortar. In addition to mechanical properties, the current use of mortars necessitates consideration of the surrounding environment, particularly in cases where they are exposes to high humidity conditions. This can arise from their application in areas with underground water or in coastal structures that are subjected to seawater immersion. The changes in the structural properties of the mortar after contact with the solution need to be considered during construction. Among the commercial polymers, epoxy resins are now extensively used because they enable the design of goods with desired features due to their exceptional qualities for enhancing strength, adhesion, durability, and water and chemical resistance.
Generally, the common epoxy materials are brittle which is an undesirable property for construction materials. The increase in the chain length and the change of chemical structure of the epoxy oligomers, both synthetic [1–3] and bio-based [4, 5] or the use of curing agents [1, 2] may improve the toughness properties of the epoxy polymer, due to the change in crosslink density and the mobility of the network structure.
According to the global waste situation, in 2021 the world had approximately 2.22 billion tons of waste, which is expected to increase to 2.59 billion tons by 2030. One of the popular plastics to be recycled is PET (e.g. PET bottles) due to its toughness and great chemical resistance. The recycling methods can be divided into 3 types: (i) primary recycling (pre-consumer scrap), which is the upcycling process of the waste, (ii) secondary recycling or physical/mechanical reprocessing, and (iii) tertiary reprocessing or chemical reprocessing, which uses a chemical process to transform the plastic waste back to its original form by using chemical processes.
Currently, there are two types of recycled PET used in combination with mortars. Firstly, secondary recycling is used in which PET is melted at high temperatures before being mixed with sand at a specified ratio. It was found that the melting temperature affected the compressive strength of the specimens [6]. Alternatively, combining ground PET with mortar has been shown to reduce compressive strength and flexural strength but increase splitting tensile strength. The combination with PET also helps mortars to achieve improved insulation properties [7]. The tertiary recycling method is used to reduce the molecular weight of PET molecules by glycolysis with glycol to achieve hydroxyl terminated PET oligomer. Epoxy resins made from hydroxyl terminated PET, amine hardener, and sand showed higher compressive strengths and flexural strengths than those made using PET obtained from secondary recycling [8, 9]. However, hydroxyl terminated PET cannot be used to cure epoxy oligomers.
The objective of this work was to create a high-performance epoxy mortar by incorporating a waste PET co-hardener and minimizing the usage of commercial amine hardener. Consequently, co-hardener with bond linkages beyond just amino groups was expected to impact the behavior of the epoxy mortar. To explore this, various ratios of amine and CTPETs, as well as different types of modified PET, were investigated. The resulting epoxy mortars were characterized of their viscoelastic behavior, physical properties, and mechanical properties. Additionally, the investigation extended studying the mechanical change before and after immersion in solutions, as well as examining the bonding strength between epoxy mortar and ordinary Portland cement (OPC).
EXPERIMENTAL
Materials
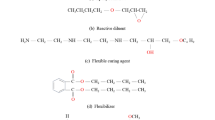
Epoxy mortar M103 (Part A: Diglycidyl ether of bisphenol A, DGEBA, 95%; EPOTEC YD 128 and Part B: amine-amide based hardener in the ratio of 75:25) was supplied by Richtech Paint Co., LTD. Three types of carboxyl terminated PET (CTPET) hardener: 1) CTPET glycolyzed with ethylene glycol (EG) and glycerol (GL) called P-EG, 2) CTPET glycolyzed with ethylene glycol (EG) and trimethylol propane (THMP) called P-ET, and 3) CTPET glycolyzed with trimethylol propane (THMP) called P-T were prepared using mole ratio of PET and glycol of 1 : 2 followed by ring opening reaction of the intermediate with phthalic anhydride (PA), were prepared in Polymer Laboratory, Naresuan University and called P-EG-PA, P-ET-PA and P-T-PA [10]. The weight average molecular weight of P-EG-PA, P-ET-PA, and P-T-PA, measured by GPC, are 733, 913, and 1.148 g/mol. Meanwhile, the Tg, midpoint values of P‑EG-PA, P-ET-PA, and P-T-PA, measured by DSC, are –31, –20, and –25°C, respectively. The chemical structures of the CTPET species are shown in Scheme 1. N,N-dimethylbenzylamine, which was used as catalyst, was purchased from Carlo Erba Reagents. The aggregate was made from commercial sand of grade 0.15–0.30 mm selected by sieve sized 50 and 100 mesh.

Scheme 1.
Epoxy Mortar
Epoxy mortar specimens were prepared in a clean container. The CTPET hardener was added in the required amount to the epoxy oligomer to give the desired weight ratio (Table 1). The mixture was stirred with a mechanical stirrer for about 15 min. and then the tertiary amine catalyst was added in a ratio of 0.25% by weight of CTPET. After stirring for 15 min, the required amounts of Part B hardener and sand were added in the mixture. The mixture was stirred for about 3 min. before pouring into a mold. Normally, all formulations of epoxy mortars can polymerize at room temperature. However, in order for the experimental results not to have any deviations due to preparation conditions, the mixtures were cured at 120°C for 2 h and then left at 35°C for 7 days before testing.
Scheme 2 presents the possible cure reaction mechanisms involving epoxy oligomer, amine, and CTPET hardener. In this study, the curing process is assumed to proceed via four curing reactions: (1) the reaction between the primary amine group and the epoxy oligomer’s oxirane ring, resulting in the formation of a tertiary amine network [2], (2) the reaction between the carboxylic acid and the oxirane ring, leading to the formation of a ester network and a hydroxyl functional group [11, 12], (3) the reaction between the carboxylic acid and the hydroxyl group, forming a polyester network [13, 14], and (4) the reaction between the carboxylic acid and the primary amine group, resulting in the formation of an amide linkage [11].

Scheme 2.
Characterization Methods
The molecular weight of the CTPET was investigated by gel permeation chromatography system (GPC, Tosoh, Japan) equipped with Tskgel G3000HXL and G2000HYL column, as well as refractometric detector. HPLC grade THF was used as the solvent, with a carrier solvent flow rate of 1 mL/min. The calibration was performed using polystyrene standards.
The glass transition temperature of the starting CTPETs was measured using a Mettler Toledo model DSC 3+ STARe system, a Differential Scanning Calorimeter (DSC). All samples were subjected to a temperature scan from –50 to 150°C at a heating rate of 10 K/min, with a constant nitrogen flow rate of 20 mL/min. Subsequently, the temperature was reduced to –50°C at the same of 10 K/min. Finally, the second heating scan was performed using the same temperature range and heating rate. Report on Tg,midpoint from second heating.
Dynamic properties of the epoxy mortar products were analyzed using the TA Instruments model DMA 850. The measurement was carried out in the dual-cantilever mode by varying the temperature from 0°C to 150°C with an increment of 3 K/min. at frequencies of 1 Hz under N2 atmosphere. Storage modulus E ', damping factor (tan δ), and crosslink density (νR) determined at elastic modulus in the rubbery state (Tg +30°C) and were calculated using the following equation [13–15]:
Compressive and three-point flexural tests were measured using an Instron Universal Tester (Model 5965 series). The testing procedure has been modified from ASTM C109 and ASTM C293 [16]. The crosshead speed was 1 mm/min. The specimens used in the compressive strength tests had dimensions of 40 × 40 × 40 mm3, while the specimens used in the flexural strength tests had dimensions of 40 × 40 × 160 mm3. The reported results represent the average data obtained from 6 specimens in each testing condition.
Water absorption and chemical resistance of epoxy mortar samples were recorded as the change of mass (Absorption) and flexural strength of the specimens exposed to various conditions. The specimens with dimensions of 40 × 40 × 160 mm3 were immersed in a given solution at RT for 7 days. The solutions were 10% w/v of H2SO4, NaOH, and NaCl [17, 18]. The average of data for 3 specimens exposed to each condition is reported. Absorption and flexural change after immersion were calculated using the following equations:
where W1 and W2 are the weights of the specimen before and after immersion in the reagent.
where F1 and F2 are the flexural strength values of the specimen before and after immersion in the reagent.
The effect of the CTPETs co-hardener on the flexural bonding strength between the commercial Portland cement mortar substrate and epoxy mortar was investigated. The ordinary Portland cement (OPC) used in this study was manufactured by Thai Pride Cement Co., LTD, Thailand and its specific gravity was 3.15. The fine aggregate was river sand. Mortar substrates were prepared with a mass proportion of cementitious binder : sand : water of 1 : 3 : 0.5 by weight. The Mortar substrates with the dimension of 40 × 40 × 160 mm were cast and kept at 35°C covered by wet cloth and plastic sheet for 24 h. After that, mortar samples were demolded and kept in the control room (temperature 28°C, relative humidity 50% RH) for 27 days [19]. In order to maintain the same surface roughness for the interface bonding performance tests, each mortar substrate sample was cut in half along the length (denoted as Cement, CO as shown in Scheme 1) and the substrate surface was then carefully ground and polished. The substrate mortar was placed into the molds before casting of the epoxy mortars into the vacant part of mold to obtain the combined specimens. In this study, seven types of co-hardeners (EA, EA-EG0.25, EA-EG0.50, EA-ET0.25, EA-ET0.50, EA-T0.25, and EA-T0.50) were used to study the interface bonding performance between the normal OPC mortar substrate (CO) and the epoxy mortar repair material. All samples were kept at 35°C for 7 days before the interfacial flexural bond strength was measured. In addition to the polymer mortar, a test specimen comprised from the OPC mortar (CN) as the repair material and the cement mortar substrate (CO) was also prepared for comparison purposes. The interfacial flexural bond strength test was performed and the results were expressed as the average of 4 values obtained from different test specimens. The dimensions and the photograph of the corresponding samples are shown in Fig. 1.
RESULTS AND DISCUSSION
Dynamic Mechanical Analysis
Viscoelastic properties of the epoxy mortar made using a co-hardener composed of an amine and CTPET depend upon many factors such as the cure behavior interactions between the epoxide and the amine, the epoxide and the CTPET and so on, type and number of functionalities of the CTPET, ratio of co-hardener, and nature of cured epoxy/aggregate interfaces.
Storage modulus E ' reflects the elastic behavior of the epoxy mortar. The \(E_{{\text{g}}}^{'}\) and \(E_{{\text{r}}}^{'}\) values describe the glassy state and the rubbery plateau and are determined at 0°C and Tg +30°C. It can be seen that the \(E_{{\text{g}}}^{'}\) of the epoxy mortar prepared with commercial amine is 21.9 GPa. The incorporation of com.amine : CTPETs in the ratio of 0.75 : 0.25 increases the \(E_{{\text{g}}}^{'}\) (Fig. 2 and Table 2) values. The \(E_{{\text{g}}}^{'}\) value presents the elastic energy stored during one cycle of oscillation or load-carrying capacities, which relates to the stiffness of the polymer chain and interfacial adhesion between the epoxy matrix and aggregates [15, 20, 21]. The results might be due to the presence of aromatic parts in the backbone of modified PET, which increase the rigidity of the epoxy mortar more than only the amine hardener alone. It was also seen that EA-ET0.25 gave the greatest \(E_{{\text{g}}}^{'}\) value followed by EA-T0.25 (with 4 COOH groups) and EA-EG0.25 (with 2–3 COOH groups). Adding excessive amounts of long chain CTPET in weight ratio of 0.75 (75%) can lead to the generation of long distances between the active sites leading to a looser network structure. This will result in a decrease in the \(E_{{\text{g}}}^{'}\) value.
The \(E_{{\text{r}}}^{'}\) values increase in the sequence EA-EG0.25, EA-ET0.25, and EA-T0.25. This could be explained by assuming that \(E_{{\text{r}}}^{'}\) depends on the crosslink density [15]. The increasing functionality of the curing agents results in increasing crosslink density. However, excessive crosslink content might affect the elastic load-carrying capacities of the material in the rubbery state. This causes EA-ET0.25, although it has lower content of crosslinking than EA-T0.25, to have greater \(E_{{\text{r}}}^{'}\) at Tg values. EA-EG0.25 with the lowest COOH content exhibited the least number of crosslinks and the lowest \(E_{{\text{r}}}^{'}\) value in comparison with the other CTPETs.
Although the combination of CTPETs and amine hardeners increases the crosslink density and rigidity of polymer chains in comparison with the amine only hardener, the longer chain length of CTPETs, in comparison with commercial amine, and lower activity of the carboxylic acid leads to lower density of epoxy mortar made with increasing CTPET ratios (0.5 and 0.75 parts). This resulted in decreased load-carrying capacity at Tg (\(E_{{\text{r}}}^{'}\)) for mortars made with these ratios of CTPETs.
The maximum tanδ (tanδmax) can be related to interfacial adhesion between the epoxy matrix and aggregate and damping properties of the material. Good damping performance materials that help to reduce noise and vibration should exhibit a high loss factor (tanδ > 0.3) over the working temperature [15, 22, 23]. However, higher peak of loss tangent represents lower interfacial adhesion between the epoxy matrix and aggregate [20, 21]. In addition, the temperature at the maximum value of tan δ is commonly identified as Tg of the polymer. The increase in Tg was attributed to the strong interactions between the epoxy matrix and aggregate that prohibit crosslink mobility of the epoxy polymer [15]. Thus, the effectiveness of the composite can be represented by low tan δ as well as high Tg values.
Epoxy mortars are commonly used for the durable floors of industrial sites and for energy damping floors. The acceptable energy damping can be characterized by tanδ > 0.3 and high epoxy-sand interaction (high Tg). EA showed tanδmax of 0.44 which corresponds to high damping properties. The incorporation of the three CTPETs resulted in higher tanδmax and also in a wider working temperature range ΔTs). The mixture of CTPETs and amine with a ratio of 0.25 : 0.75 exhibited tan δmax in the range of 0.47–0.50 and the highest Tg in comparison with other ratios of the co-hardener. EA-T0.25 exhibited minimum tanδ (0.47) and maximum Tg (64.45°C) values associated with the highest interaction between the epoxy and sand components and energy damping of the sample. Decreases in Tg values were observed in EA-ET0.25 and EA-EG0.25.
In addition, it can be seen from Fig. 2 that the tanδ values of epoxy mortars made using amine hardener and amine/CTPET hardener showed single tanδ peak, which indicates good compatibility between the epoxy matrix and aggregate [21, 24]. Although a single peak was observed, the incorporation of CTPETs caused broader tanδ peak indicating development of heterogeneity in the reaction of different hardeners [24]. Regarding the temperature range leading to good damping properties (tan δ > 0.3), the amine hardener only caused the maximum damping behavior in the range of 36–58°C. The incorporation of 25% of a CTPET co-hardener resulted in a broader ΔT at tanδ > 0.3 (Table 2). This result might be due to the interpenetration network formed between the epoxy oligomer and the amine and epoxy oligomer and CTPETs leading to broader damping properties at higher temperatures. A higher content of a CTPET co-hardener (especially in 75%) resulted in higher tanδmax and narrower ΔT. The increase in tanδmax is associated with decreased interaction between the epoxy matrix and sand. It could clearly be seen that EA-EG0.75, which contains 2–3 COOH groups exhibited the greatest tan δmax corresponding to the lowest interaction between the epoxy and sand.
Mechanical Properties
Figure 3 shows the compressive and flexural strength of epoxy mortars prepared using different types of co-hardener. The compressive strength of the epoxy mortar with part B hardener (EA) is 52 MPa. Blending a CTPET co-hardener with the epoxy mortar up to a content of 50% causes a further increase in compressive strength. The highest compressive strength obtained with EA-T0.25 co-hardener is 81 MPa. This could be due to the highest crosslink density, four functionalities, and hidden structure with the spatial engagement of ethyl and aromatic groups in the backbone, which increase the rigidity and compressive strength of the epoxy mortar [1]. EA‑ET0.25 exhibits crosslink density similar to EA‑T0.25, however it’s three functionalities and flexible ethylene oxide cause lower Tg and strength values. On the other hand, the EA-EG0.25 resulted in the lowest strength among the co-hardeners containing 25% of CTPETs. The low strength of EA-EG0.25 may be due to the lowest crosslink density and increased steric hindrance in the backbone of the molecule in comparison with the ET and T structures. However, a looser nature of the CTPET network and decrease of the interfacial adhesion between the epoxy and aggregate particles will be reduced once the CTPET ratio is raised over 50% [1, 21].
Flexural strength of neat epoxy mortar (EA) is 29 MPa as shown in Fig. 3. The partial replacement of part B hardener with 25% of a CTPET as a co-hardener causes a further increase in flexural strength. The EA-T0.25 mixture results in the highest flexural strength, which was found to be 38 MPa (up to 28%). In the case of the EA-ET0.25 samples, the increase in flexural strength of epoxy mortars reaches up to 21% in comparison to the EA mixture while it is about 9% for the EA-EG0.25 mixture. The improvement in flexural strength of the epoxy mortar with 25% of CTPET co-hardener may be due to the increased crosslink density and interfacial adhesion between the epoxy and aggregate [1]. However, the flexural strength of the epoxy mortar is less than that of the control specimens (EA) when more than 50% of part B hardener is replaced with a CTPET co-hardener and it decreases with increasing CTPET co-hardener content. In addition, in comparison with the control, the flexural strain increased from 1% to 2–6% with increasing content of CTPETs up to 50%, which was attributed to increased energy absorption by the CTPETs [25]. However, further increase in the content of the CTPETs (75% of CTPETs) results in low interfacial adhesion between the epoxy and aggregate, leading to a lower failure strain [1, 20, 24].
Water Absorption and Chemical Resistance
Permeability of water and solutions are important factors for induced corrosion of construction materials that affect the strength of building structures. It can be observed that the weight change after 7 days of immersion, the absorption of neat epoxy mortar in all solutions was lower than for mortars made the addition of a CTPET co-hardener (Fig. 4). The absorption values increased with the increasing CTPET content especially in water and basic solutions [18]. The absorption increases from 0.16% to 0.7–0.9, 1–1.1, and 1.7–1.8% of water and from 0.14 to 0.65–0.86, 2.0–2.4, and 4.2–4.8% in 10% NaOH with the addition of 25, 50, and 75% of a CTPET co-hardener, respectively. This may be because of the looseness of the structure of the epoxy matrix that was cured with CTPET [8] and the interactions between the ester groups of the CTPET and the hydroxyl groups of the solution.
EG with the least number of functionalities was found to exhibit the highest solution absorption while ET and T with 3 and 4 functionalities exhibited similar absorption, which was less than that of EG. In addition, epoxy mortars cured with all CTPETs presented great prevention of the absorption of 10% H2SO4 and 10% NaCl with less than 1% adsorption in both solutions i.e. 0.39–0.82%. While, special amine-CTPET with 25% gave less than 1% absorption of H2O and 10% NaOH.
Figures 5 and 6 show the flexural strength and flexural change before and after immersion of epoxy mortar in water and various solutions for 7 days. Water absorption by neat epoxy mortar (0.14%) was significantly lower than for amine-CTPET cured mortars (0.78–0.98%). However, measurements of flexural strength and flexural change, especially for CTPET content of 25%, showed the same 4–5% decrement in values in comparison with values before immersion for all samples. In addition, similar trends in the change in flexural values were observed for different amounts of functionality present in the CTPETs.
In 10% NaCl solution, the 25% content of CTPETs enhanced resistance to chloride corrosion. The mixed mortar specimens showed an increase in flexural strength in comparison to the neat epoxy mortar as seen in Fig. 5. While only T-0.25 showed an increase in flexural strength, which presents similar results as neat epoxy mortar after 10% H2SO4 immersion. However, when comparing the flexural change after immersion in 10% NaOH, the flexural strength of the immersed neat epoxy mortar increased, while for mortars with added content of CTPET it decreased, especially for CTPETs ratio higher than 50%. This may be due to the penetration of NaOH potentially leading to the hydrolysis of ester bonds of the CTPETs, resulting in the destruction of the crosslinked structure of the epoxy matrix and deteriorating flexural properties [18].
Interface Bonding Performance
In this study, the interfacial flexural strength was used to assess the interface bonding performance between the prepared epoxy resin and normal OPC mortar as the substrate material. Table 3 shows the interface morphology of test samples based on the optical microscope (OM) images with a 5× magnification. The images show that for the studied specimens, there was no visible crack in the interface between the OPC mortar substrate and the epoxy mortar before the applying of loads. However, a thin crack was found near the interface of the OPC mortar repair material (CN) and the mortar substrate (CO). This may be due to the shrinkage of these cement mortars. The failure of all composite specimens made with the epoxy mortar and the substrate occurred within the mortar substrate near the middle of the specimen, without debonding or cracks at the interface. The flexural strength of the OPC mortar specimen (CO), the OPC mortar substrate bonded with the new cement mortar (CO:CN) and the mortar substrate/epoxy mortar composites (CO:EA, CO:EA-EG, CO:EA-ET and CO:EA-T) are presented in Fig. 7. It is obvious that all composite samples made using the epoxy mortar repair material displayed higher flexural strength compared to the CO:CN composite specimen regardless of different hardener contents. The highest value of 5.7 MPa was observed for the CO:EA-ET0.25 mortar, followed by 5.5, 4.8, and 4.6 MPa for the CO:EA-T0.25, CO:EA, and CO:EA-EG0.25 mortars, respectively. It can clearly be seen that in comparison to the CO and CO:CN specimens, epoxy mortar used in this study has a strong adhesion with the mortar substrate and therefore it can be effectively used as the bonding agent in concrete repair application. Since the failure mode occurs within the mortar substrate near the interface region, the higher flexural bond strength of the CO:EA-ET0.25 and CO:EA-T0.25 composite specimens compared to the control specimens (CO:EA) can be mainly due to the improvement of the flexural strength of the cement mortar near the interface region by the infiltration of the polymer film into the small pores or gaps on the surface of the mortar substrate [26–28]. This polymer film improves the interface between the mortar binder matrix and the fine aggregate leading to higher flexural strength of the cement mortar near the interface. This can indicate that the high content of carboxylic acid functionalities of the CTPETs helps to improve the interface bonding performance of repaired cement better than only 2 acid functionalities of CTPET and the commercial amine hardener. However, when the amount of replacement ratio of CTPETs increases to 50%, the flexural bond strength of composite samples decreases by values in the range 23–30%. This may be because of the decrease of crosslink density and interfacial adhesion behavior between the epoxy, aggregate particles, and old ordinary cement-based will result in reduced interfacial flexural bonding strength of the repaired material [1].
CONCLUSIONS
This research study investigates the viscoelastic and mechanical properties of epoxy mortars incorporation a combination of amine, amide, and ester linkages from amine-amide and CTPETs hardeners. The main conclusions are as follows:
1. The inclusion of a CTPET co-hardener effectively enhances the viscoelastic behavior and mechanical properties of the epoxy mortar. The optimized epoxy mortar made with 25% P-T-PA co-hardener exhibited the best enhancement effect, which can be attributed to the higher concentration of rigid aromatic groups and improved interactions between the epoxy and sand.
2. The addition of CTPET co-hardeners, especially those with 3 or 4 acid functionalities, in an appropriate proportion, enhances the interfacial flexural bonding performance. However, excessive content of CTPETs in the epoxy mortar lead to reduced compressive and flexural strengths, as well as decreased flexural bonding strength for all CTPETs. This decline may be attributed to the loss of ester linkage points and reduced adhesion between the epoxy and aggregate.
3. Epoxy mortar cured with 0.25% P-T-PA co-hardener demonstrates excellent corrosion resistance in acid and chloride solution. It exhibits improved flexural strength compared to its pre-immersion state, particularly in 10% NaCl, outperforming the neat epoxy mortar.
4. The presence of ester linkage in epoxy mortar formulated with a CTPET co-hardener results in reduced flexural strength after immersion in H2O and 10% NaOH, owing to the hydrolysis of the ester bonds in the CTPETs.
REFERENCES
E. Ozeren Ozgul and M. H. Ozkul, Constr. Build. Mater. 187, 360 (2018).
F. L. Jin, X. Li, and S. J. Park, J. Ind. Eng. Chem. 29, 1 (2015).
N. J. Jin, J. Yeon, I. Seung, and K. S. Yeon, Constr. Build. Mater. 156, 933 (2017).
S. Ma, T. Li, X. Liu, and J. Zhu, Polym. Int. 65, 164 (2016).
P. Ruamcharoen, S. Umaree, and J. Ruamcharoen, J. Mater. Sci. Eng. 5, 504 (2011).
Z. Ge, R. Sun, K. Zhang, Z. Gao, and P. Li, Constr. Build. Mater. 44, 81 (2013).
G. Lazorenko, A. Kasprzhitskii, and E. H. Fini, J. Clean. Prod. 375, 134083 (2022).
B. Dębska and L. Lichołai, Constr. Build. Mater. 94, 579 (2015).
B. Dębska and L. Lichołai, Constr. Build. Mater. 124, 11 (2016).
C. W. Phetphaisit, R. Bumee, J. Namahoot, J. Ruamcharoen, and P. Ruamcharoen, Int. J. Adhes. Adhes. 41, 127 (2013).
V. Rebizant, A. S. Venet, F. Tournilhac, E. Girard-Reydet, C. Navarro, J. P. Pascault, and L. Leibler, Macromolecules 37, 8017 (2004).
D. Foix, Y. Yu, A. Serra, X. Ramis, and J. M. Salla, Eur. Polym. J. 45, 1454 (2009).
S. J. Park and F. L. Polym. Degrad. Stab. 86, 515 (2004).
F. Mustata and N. Tudorachi, Appl. Therm. Eng. 125, 285 (2017).
Y. Chen, N. Hossiney, X. Yang, and H. Wang, Adv. Mater. Sci. Eng. 2021, 3454029 (2021).
M. T. Lakhiar, Y. Bai, L. S. Wong, S. C. Paul, V. Anggraini, and S. Y. Kong, Constr. Build. Mater. 315, 125677 (2022).
B. Debska and L. Licholai, Constr. Build. Mater. 65, 604 (2014).
M. C. S. Ribeiro, C. M. L. Tavares, and A. J. M. Ferreira, J. Polym. Eng. 22, 27 (2002).
L. K. Aggarwal, P. C. Thapliyal, and S. R. Karade, Constr. Build. Mater. 21, 379 (2007).
O. Y. Alothman, M. Jawaid, K. Senthilkumar, M. Chandrasekar, B. A. Alshammari, H. Fouad, M. Hashem, and S. Siengchin, J. Mater. Res. Technol. 9, 15537 (2020).
R. Senthilkumar, M. P. Natarajan, S. Ponnuvel, and R. Sathyamurthy, J. Mater. Res. Technol. 17, 819 (2022).
H. Yin, H. Jin, C. Wang, Y. Sun, Z. Yuan, H. Xie, Z. Wang, and R. Cheng, J. Therm. Anal. Calorim. 115,1073 (2014).
X. Zhou, G. Zhang, W. Zhang, W. Guo, and J. Wang, Polym. J. 44, 382 (2012).
N. Z. Tomic, M. Saeedifar, M. N. Saleh, A. Marinkovic, D. Zarouchas, and S. Texixeira de Freitas, Polym. Test. 106, 107444 (2022).
S. Srisuwan, N. Prasoetsopha, N. Suppakarn, and P. Chumsamrong, Energy Proced. 56, 19 (2014).
S. Y. Guo, X. Zhang, J. Z. Chen, B. Mou, H. S. Shang, P. Wang, and L. J. R. Zhang, Constr. Build. Mater. 264, 120715 (2020).
Z. Zheng, Y. Li, S. He, X. Ma, X. Zhu, and S. Li, Constr. Build. Mater. 197, 319 (2019).
S. Y. Guo, X. Zhang, J. Ren, J. Z. Chen, T. J. Zhao, T. W. Li, and L. Zhang, Constr. Build. Mater. 272, 121960 (2021).
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phetphaisit, C.W., Singthong, W., Hemavibool, S. et al. Viscoelastic and Mechanical Properties of Repair Epoxy Mortar from Modified Poly(ethylene terephthalate) Waste. Polym. Sci. Ser. A 65, 568–579 (2023). https://doi.org/10.1134/S0965545X23701183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965545X23701183