Abstract
New modifications of a polyampholyte sorbent based on carbamide (urea), formaldehyde, and aminoacetic acid, differing in the reagents ratio during the synthesis, have been prepared. It has been shown that the optimal synthesis temperature is 90°C. Thermal stability of the polyampholyte has been analyzed. Sorption properties of the polyampholyte towards d-element ions have been investigated. The effect of the synthesis temperature on specific volume and static exchange capacity of the polyampholyte has been considered. The influence of the medium pH on sorption of copper(II) ions with the polyampholyte has been evaluated. It has been found that the sorption degree by the considered sorbents is the highest at pH from 5.5 to 6.5. The polyampholyte prepared at the urea, formaldehyde, and aminoacetic acid ratio 2 : 5 : 0.7 has revealed the highest sorption capacity towards the d-element ions. The sorption rate has been maximum at the solution temperature of 20°C. The adsorption has been shown to follow the Freundlich equation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Many natural polymers and synthetic substances exhibit ion-exchange properties [1]. The most important of them are ionites based on synthetic polymers [2, 3], also known as polyampholytes or ion-exchange resins.
Significant progress has been made in recent years in the production of polyampholytes. However, many of them, especially these of the polycondensation type, still do not meet the requirements for availability, efficiency, and sorption capacity and selectivity, which leads to the need for synthesis of novel ion-exchange polymers [4]. In this regard, the development of new polyampholytes and improvement of their production processes are given great attention worldwide [5]. Large-scale use of ion-exchange materials in various areas of industrial production stimulates further improvement of the processes for their production [6]. Moreover, the use of ionites increases the yield of products in various processes, thus reducing the energy and material costs and simplifying the technological schemes.
The ionite adsorbents have also proven to be substances used to reduce the environmental impact, for instance, to purify wastewater from poisonous ions [7, 8].
Polymer sorbents exhibiting chelating properties are widely used in modern wastewater treatment technologies [9]. The search for new ion exchangers with increased thermal and chemical resistance as well as selectivity to ions of technologically important rare and non-ferrous metals (molybdenum, copper, cobalt, nickel, and others) is among the urgent tasks of modern science and industry [10]. In general, the modification of ion-exchange materials is currently an extremely intensively developing branch of modern chemistry. However, despite the recent advances, the issue of preparation of polyampholytes has remained among the most relevant.
A chelate-forming sorbent based on urea, formaldehyde, and hydrazine hydrate has been earlier synthesized, and the process of its complex formation with Cu(II), Zn(II), and Cd(II) ions has been investigated [11]. Furthermore, the process of covalent attachment of these ions at the matrix of urea-formaldehyde resin with 2-aminopentanedioic (glutamic) acid (sorbent UFG) [12] and dithizone (sorbent UFD) [13] has been studied. It has been found that these polyfunctional anionites exhibit improved ion-exchange properties towards ions of such salts as sulfates, nitrates, and chlorides [14].
Our ultimate goal is to obtain a wide range of different polyampholytes capable of adsorbing metal ions from solutions. In the future, it is planned to evaluate the possibility to use these polyampholytes as adsorbents for microplastic particles that pollute the environment, especially water [15]. It has been found in a number of studies that the micro- and nanoplastic particles are clearly detected in various natural objects and food environments such as water [16] and vegetable oils (during storage) [17] and are even readily released from pyramidal tea bags during brewing [18]. In this regard, the development of adsorbents capable of removing both heavy metal ions and microplastics is very important.
As a result of earlier studies [19], a polyampholyte sorbent based on urea, formaldehyde, and aminoacetic acid (UFA) has been obtained. The effect of the medium pH and temperature on its synthesis has been elucidated, the IR spectra have been analyzed, elemental analysis of the synthesized complexes has been performed, and their surface structure has been probed by means of scanning electron microscopy. Polyampholyte prepared via polycondensation of o-aminobenzoic acid and epoxy resin bearing the Bisphenol A moieties has been recently studied by us [20].
In the present study, adsorption parameters of different modifications of polyampholyte sorbent synthesized via polycondensation of urea-formaldehyde resin with aminoacetic acid were determined. The study aimed to investigate sorption properties of those materials towards copper(II) ions. The mechanism of Cu2+ sorption by the polyampholyte adsorbents was also analyzed. It has been earlier found basing on the isothermal, kinetic, and thermodynamic experiments that the process of uranium sorption by chelating polymers follows the Langmuir model with pseudo second-order mechanism and exothermic nature [21]. In the present study, the sorption process was analyzed using the Langmuir and Freundlich isotherms.
EXPERIMENTAL
The modifications of the considered sorbent were obtained via polycondensation from carbamide (urea), formaldehyde, and aminoacetic acid as described elsewhere [19], at different ratios of the reagents.
Thermoanalytical experiments were performed using a Netzsch STA 409 PG analyzer (Germany) with a type K (Low RG Silver) thermocouple and aluminum crucibles. The measurements were performed under inert nitrogen atmosphere (flowrate 50 mL/mol). The temperature range was 25–370°C, heating rate being 5 deg/min. A specimen mass was 5–10 mg. The instrument was calibrated using a set of references: KNO3, In, Bi, Sn, and Zn.
Specific volume of the swollen sorbents was determined according to GOST 10898.4–84, and static exchange capacity was determined according to GOST 20255.1–89 “Ionites. Methods to Determine Static Exchange Capacity”, by analogy with [22]. The chemicals of pure and chemical pure grades were used. The solutions were prepared via dissolution of a weighed portion of the chemical in a known volume of the solvent.
For the synthesized complex-forming UFA sorbents, isotherms of adsorption of the metal ions (Cu2+, Co2+, Cd2+, Zn2+, and Ni2+) were investigated. To do so, aqueous solutions of salts of the said metals with concentration of 0.1 eq/L (volume 10 mL) were prepared. Then, 0.5 g of the granulated UFA sorbent was added to each solution. The sorbent was kept in the solutions during 4 h, for the metal sorption to occur. The solution color and the sorbent were changed due to the metal ions transition into the adsorbed state. The sorbent complexes with the metals were then separated from the solutions. As a result, swollen multi-colored granules were obtained, which were dried in an oven at room temperature.
To determine the adsorption capacity (static exchange capacity) towards the ions, the metal concentration in the solution was measured before (0.1 N in each case) and after the adsorption by means of spectrophotometry or complexometric titration.
Optical path length during the spectrophotometric measurement was 5 cm. In the case of Cu2+ ion, forming dark blue ammonia complexes with a dilute ammonia solution, the absorbance was measured at 610 nm. In the case of Ni2+ ion, forming blue-violet complexes with dilute ammonia solution, the measurements were performed at 565 nm. Determination of the Со2+ ions were performed upon formation of yellow complex with murexide from the initially pink solution (measurements at 460 nm). Concentration of Cd2+ and Zn2+ was determined by complexometric titration, as described in [23] and [24], respectively. In that case, solutions of dithizone with complexon III were used.
Static exchange capacity (SEC) was calculated via the equation
with с0 being the ions concentration before adsorption (0.1 N), с being the ions concentration upon adsorption, and m being bulk mass of the adsorbent. According to GOST 20255.1-89, the resulting SEC of the ionite was determined as mean of two independent measurements if the |SEC-1 – SEC-2| < 0.025(SEC-1 + SEC-2)/2, condition was fulfilled, with SEC-1 and SEC-2 being the results of independent measurements of static exchange capacity and 0.025 (2.5%) being the repeatability limit at probability Р = 0.95.
RESULTS AND DISCUSSION
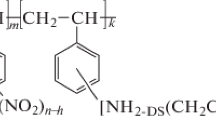
The suggested structure of the polyampholyte sorbent based on carbamide (urea), formaldehyde, and aminoacetic acid is presented below.

Earlier [19], we obtained the UFA polyampholyte at the molar ratio of the reactants (urea : formaldehyde : aminoacetic acid) equal to 2 : 5 : 0.2 (UFA-0.2). In the present study, in order to improve the adsorption capacity of UFA, we prepared three new polymers of the modified composition, at the reactants molar ratio 2 : 5 : 0.5 (UFA-0.5), 2 : 5 : 0.7 (UFA-0.7), and 2 : 5 : 1.0 (UFA-1).
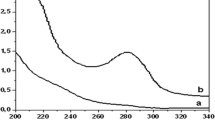
First, the thermal stability of the UFA-0.2 polyampholyte was investigated. According to the TGA and DTA data (Fig. 1), onset of the sorbent melting occurred at 200°C. The endothermic peak was observed at 246–335°C. The polyampholyte decomposition started at 290°C. The mass loss at 220–340°C equaled 84.6%. From the obtained data, it could be concluded that the mass loss was due to elimination of water and decomposition of various functional group. A similar picture has been observed earlier in the thermogravimetric analysis data of polyampholyte sorbent based on urea, formaldehyde, and dithizone [13].
Further, in order to optimize the process of synthesis of the polyampholyte, we investigated the influence of temperature on the process of polycondensation of urea, formaldehyde, and aminoacetic acid in the case of the UFA-0.2 polymer. The polycondensation was performed at 75, 85, 90, 100°C. The completion of the reaction was judged by the transformation of the liquid mixture into a resinous mass.
The dependence on the static exchange capacity with respect to 0.1 N NaOH solution and specific volume of the water-swollen sorbent on the duration of the reaction was also investigated. The results are given in Table 1. It is to be seen that at low temperature (75°C) the polycondensation reaction was complete in 3 h, exchange capacity of the copolymer being 2‒2.5 meq/g. The reason for long duration of the reaction was low reactivity of the compounds at that temperature. When the reaction temperature was increased to 100°C, the crosslinking process was faster and the reaction duration was decreased to 1 h, but exchange capacity and swelling ability of the copolymer were decreased as well. Probably, structure of the copolymer formed at higher temperature was denser, and mobility of the ionic groups was hindered.
Therefore, the optimal temperature to conduct polycondensation was 90°C: the reaction duration at that temperature equaled 1.5‒2.0 h, the reaction course was more uniform, and the exchange capacity with respect to 0.1 N solution of NaOH was as high as 4.1 meq/g. The polyampholytes with different ratios of the reactants (UFA-0.5, UFA-0.7, and UFA-1) were further synthesized under the chosen conditions.
For the new modifications of the UFA polyampholyte, the dependence of static sorption capacity towards copper(II) ions on the medium pH was determined (Fig. 2). As follows from the data in Fig. 2, sorption capacity of the adsorbents with respect to Cu2+ ions was the highest at рН of 5–6. The sorption capacity of UFA-0.5 at pH 5.6 equaled 4.3 meq/g, being 4.6 meq/g for UFA-0.7 (рН 6.0) and 4.4 meq/g for UFA-1 (рН 6.0). It was found that the UFA-0.7 exhibited higher sorption capacity towards copper(II) ions than other sorbents (UFA-0.2 [19], UFA-0.5, and UFA-1). In a weakly acidic medium (рН 5–6), Cu2+ ions were adsorbed due to the complex formation with protonated functional groups. Deterioration of the sorption properties of UFA-1.0 compared to UFA-0.7 could be explained by the fact that the increase in the amount of the functional groups in the polyampholyte was leveled by compaction of the sorbent structure and decrease in the distance between the functional groups, as well as the decrease in the sorption ability due to hindered diffusion of the ions in the inner sphere of the polymer.
Once the optimal pH for operation of the polyampholyte adsorbents was found, it was possible to turn to investigation of adsorption of ions of other d-elements: Zn2+, Ni2+, Co2+, Cd2+. Its results (dependence of the sorption properties of the ionite on the components ratio during its synthesis) are given in Table 2.
In view of the repeatability limit of 2.5%, the difference in the SEC of certain ions (for example, Zn2+ and Co2+) could be considered statistically insignificant, and the adsorption capacity of different modifications of UFA was the same. However, in the case of Cu2+ and Ni2+, the values of SEC of different modifications of UFA were different by 5–10%. Based on the obtained data, UFA-0.7 was the best adsorbent.
The data in Table 2 revealed that the increase in the content of aminoacetic acid initially to the increase in the exchange capacity, followed by its decrease. Such behavior could be explained by the decrease in the pore radius of the ion exchanger and the appearance of geometrical obstacles for the ions diffusion as well as decrease in the swelling degree. The performed experiments revealed that the ion exchangers with the best characteristics were obtained at the urea, formaldehyde, and aminoacetic acid ratio of 2 : 5 : 0.7 (UFA-0.7).
Sorption of Cu2+ with the most efficient polyampholyte UFA-0.7 at different temperatures was analyzed using Langmuir and Freundlich isotherms. The change in the solution concentration due to sorption of Cu2+ ions with the polyampholyte was monitored. The sorption experiment was performed at 20, 40, 60, and 80°C. Efficiency of sorption a (mol/g), i. e., the amount of the component in the ionite phase was calculated using the equation
with V being the solution volume, L; g being the ionite mass, g; С0 being the initial component concentration, mol/L; С being the component concentration upon the sorption, mol/L.
Equations for the Langmuir and Freundlich adsorption isotherms are as follows:
and
respectively. Here, KL and KF are the equilibrium constants, Г∞ is the maximum capacity of the ionite, mol/g; C is the equilibrium concentration of the component in the solution, mol/L; n is the Freundlich isotherm parameter reflecting the sorption efficiency. The parameters of those equations were determined from the experimental data via the least squares method implemented in Microsoft Excel 2013 software.
From the isotherms in Figs. 3 and 4 it is to be seen that sorption of Cu(II) by the UFA-0.7 ionite followed the Freundlich model. The calculated parameters of the isotherms of Cu(II) ions sorption are collected in Table 3. From the data in Table 3 it is to be seen that the equilibrium constant KF ranged between 2.5 and 1.6. Hence, it could be concluded that the ligands were selective towards Cu(II). The increase in the calculated maximum sorption capacity of the sorbent with the decrease in temperature evidenced the stability of the coordination bonds formed by the ion. At lower temperature, the solubility of the sorbent was increased, hence the adsorbed ions could penetrate deeper into the ligand. According to the results, 20°C turned out to be optimal temperature for sorption of Cu2+.
Isotherms of Cu2+ ions sorption by UFA-0.7 in the coordinates of the Langmuir model. Т = 20 (1), 40 (2), 60 (3), and 80°С (4). Here and in Fig. 4 the lines are linear approximations of the experimental data.
CONCLUSION
In the present study, new modification of polyampholyte based on carbamide (urea), formaldehyde, and aminoacetic acid were prepared for the first time at different ratios of the reactants: UFA-0.5, UFA-0.7, and UFA-1.0. Thermogravimetric investigation of the UFA-0.2 polyampholyte was performed, and features of its thermal decomposition were found. Adsorption parameters of the UFA-0.2 polyampholyte were studied, and its static exchange capacity depending on the synthesis conditions was determined. The optimal conditions to prepare the UFA polyampholytes were determined: polycondensation temperature 90°C and the reaction duration 1.5–2 h. Static exchange capacity of the UFA-0.5, UFA-0.7, and UFA-1.0 polyampholytes was measured, and their sorption properties with respect to heavy metal ions were characterized. The best properties were observed for the polymer obtained at the urea, formaldehyde, and aminoacetic acid ratio of 2: 5: 0.7 (UFA-0.7). The process of sorption of Cu2+ ions by the UFA-0.7 polyampholyte was analyzed using the Langmuir and Freundlich isotherms. The sorption of Cu2+ ions obeyed the Freundlich model. Parameters of the sorption isotherms in the scope of that model were calculated.
REFERENCES
V. Beaugeard, J. Muller, A. Graillot, X. Ding, R. Jean-Jacques, and S. Monge, React. Funct. Polym. 152, 104599 (2020).
E. K. Spirin and L. V. Mis’kevich, Privolzhskii Nauch. Vestn. 20 (4), 5 (2013).
D. Prabhakaran and M. S. Subramanian, Talanta 59 (6), 1227 (2003).
F. V. Igitov, M. I. Berdyeva, Sh. A. Mulatov, S. M. Torobzhonov, T. T. Tursunov, and R. A. Nazirova, Sovr. Materialy, Oborudovanie I Tekhnologii Polikondensatsii 7 (4), 80 (2016).
S. V. Saikova, G. L. Pashkov, and M. V. Panteleeva, Zhurn. Sibirskogo Federal’Nogo Un-Ta. Tekhnika I Tekhnologii, No. 4, 482 (2015).
A. S. Obodovskii, N. V. Balanovskii, Yu. M. Averina, and A. G. Cherednichenko, Uspekhi V Khimii I Khim. Tekhnologii, No. 2, 96 (2016).
M. A. Barakat, Arab. J. Chem., No. 4, 361 (2011).
A. Ihsanullah, A. M. Abbas, T. Al-Amer, M. J. Laoui, M. S. Al-Marri, M. Nasser, and M. A. Khraisheh, Sep. Purif. Technol., No. 157, 141 (2016).
M. A. Babiev and S. M. Uvasova, Nauch. Zhurn. Kubanskogo Gos. Agrarnogo Un-Ta 4 (118), 1 (2016).
Kh. L. Pulatov and S. M. Turabzhanov, Universum: Tekhnicheskie Nauki, No. 12, 29 (2016).
Sh. A. Kasimov, Kh. Kh. Turaev, and A. T. Dzhalilov, Universum: Khimiya I Biologiya. Elektron. Nauchn. Zhurn, No. 3, 17 (2018).
N. A. Ermuratova, Sh. A. Kasimov, and Kh. Kh. Turaev, Universum: Tekhnicheskie Nauki, No. 4, 71 (2021).
N. Chorieva, N. Ermuratova, Kh. Kh. Turaev, and Sh. A. Kasimov, Izv. Vyssh. Uchebn. Zaved., Khim. Tekhnol., No. 4, 19 (2020).
F. B. Eshkurbonov, K. K. Turaev, and N. A. Ermuratova, Universum: Tekhnicheskie Nauki, No. 4, 49 (2019).
S. E. Nelms, T. S. Galloway, B. J. Godley, D. S. Jarvis, and P. K. Lindeque, Environ. Pollut., No. 238, 999 (2018).
R. Al-Jaibachi and R. N. Cuthbert, Biol. Lett., No. 14(4), 1 (2018).
K. N. Kornilov and N. N. Roeva, Health, Food and Biotechnology, No. 2(1) (2020).
L. M. Hernandez, E. X. Genbo, C. E. Larsson, R. Tahara, B. Vimal, and N. Tufenkji, Environ. Sci. Technol. 53 (21), 12300 (2019).
N. A. Ermuratova, Kh. X. Turaev, K. N. Kornilov, and N. N. Roeva, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 65 (9), 31 (2022).
G. A. Umirova, Kh. Kh. Turaev, K. N. Kornilov, and N. A. Ermuratova, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 66 (5), 41 (2023).
A. A. Younes, A. M. Masoud, and M. H. Taha, Sep. Sci. Technol. 53 (16), 2573 (2018).
M. Zh. Abduvalieva, Sh. A. Kasimov, Kh. Kh. Turaev, and M. N. Sharofov, Universum: Tekhnicheskie Nauki, No. 11, 92 (2021).
L. Yu. Dzhamolova and G. S. Turaeva, Vestn. Nauki I Obrazovaniya, No. 3, 134 (2023).
T. I. Akhmetova, Vestn. Kazanskogo Tekhnol. Un-Ta, No. 12, 59 (2013).
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Karpushkin
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ermuratova, N.A., Turaev, K.K., Kornilov, K.N. et al. Adsorption Ability of Nitrogen-Containing Polymer Sorbents Based on Urea-Formaldehyde Resin and Aminoacetic Acid Towards Heavy Metal Ions. Polym. Sci. Ser. A 65, 666–671 (2023). https://doi.org/10.1134/S0965545X23600679
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965545X23600679