Abstract
The effect of low-frequency acoustic treatment and of constant and alternating electromagnetic fields on the reactivity of crude oil and resin–asphaltene components was studied. Irrespective of the kind of the wave treatment, the amount of asphaltenes and resins separated from the treated oil decreases owing the breakdown of aggregates of complex structural units with the release of hydrocarbons of various structures, occluded in the molecular complexes, into the liquid phase. The treatment involves cleavage of weak hydrogen bonds with the formation of additional reaction sites in paramagnetic asphaltenes and diamagnetic resins. The reactivity of both crude oil and the separated resin and asphaltene fractions significantly changes owing to the formation of new reactive structures in the physical fields. These structures differ not only in the size and structure but also in the antioxidant properties. The data obtained allow more detailed evaluation of the effect exerted by various kinds of wave treatment on the composition and structure of asphaltenes and resins of heavy high-viscosity crude.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
The action of physical fields of various types on petroleum-containing systems (crude oils, petroleum emulsions, petroleum slimes, petroleum products, etc.) to alter their properties attracts stable researchers’ interest because it allows directional rearrangement of the structure, taking into account the individual features and using the properly chosen treatment type. For example, the viscosity–temperature characteristics of crude oils with different content of high-molecular-mass components (waxes, resins, and asphaltenes) can decrease in electric [1, 2], electromagnetic [3, 4], and magnetic fields [5–8]. The modern technologies using acoustic fields of various intensities [9–11] ensure significant breakdown of the structure of heavy crudes, petroleum slimes, and water–oil emulsions with minimal power consumption. It seems promising to study how the viscosity of heavy crudes decreases under the action of microwave and laser radiations [12, 13].
In accordance with the modern colloid-chemical concepts, the properties of a petroleum disperse system (PDS) are determined by the structure, size, and composition of a complex structural unit (CSU) consisting of a polar core formed by paramagnetic asphaltenes, less polar intermediate layer, and low-polarity shell, both formed by resins [14]. The presence of stable radicals and tetravalent vanadium complexes in resin–asphaltene components (RACs) is responsible for the paramagnetic properties of crude oil.
The mechanisms of structure formation in PDS under the action of external factors and the relationship between these processes and viscosity–temperature characteristics are widely discussed in the literature [1, 4–6, 10, 15, 16]. As has been shown, the action of an electric, ultrasonic, or magnetic field can lead to cleavage of weak intermolecular or hydrogen bonds in molecular complexes (clusters), which, in turn, will lead to an increase in the concentration of aromatic and saturated hydrocarbons in the dispersion medium. Their release from PDS clusters is accompanied by a decrease in the viscosity–temperature parameters.
The effect of physical fields on the structural-group parameters of petroleum-containing systems (PSs) can be estimated from changes in the kinetic characteristics of natural petroleum antioxidants (AOs), or inhibitors of radical-chain oxidation processes. It is known that the antioxidant activity (AOA) is determined by the activity of compounds with functional groups, containing a mobile hydrogen atom, and by processes based on intermolecular interactions leading to associative transformations via release or binding of reaction sites [17].
Oxidation inhibitors, or AOs, are used as components of many petroleum products, e.g., gasoline, motor, and diesel fuels, road asphalts, lubricating oils, consistent lubricants, and drilling fluids, and also as stabilizers for various synthetic rubbers and for polypropylene fibers. In addition, natural AOs are widely used as additives to foodstuffs containing fats and vitamins, in production of forage for animals, and in pharmaceutical and cosmetic industry [18].
A study of the AO distribution in crude oil has shown that the major fraction of AOs is concentrated in high-molecular-mass compounds (HMMCs), namely, in asphaltenes and resins [19]. The reactive sites in RACs are usually functional groups sterically shielded in arene, cycloalkane, and heterocyclic fragments, and also free radicals [20, 21]. Molecules of petroleum HMMCs contain a wide set of fragments responsible for the thermostabilizing and antioxidant properties of PSs. These are various oxygen-containing groups bonded to the aromatic core (phenol, acid, ether, ester), sulfur-containing fragments (thiol, thiophene, sulfride, thiacyclane), basic and neutral nitrogen-containing compounds, polycycloaromatic cores, and paramagnetic species or free radicals that are formed by oxidation of inhibitors (phenols and amines) and contain one or several unpaired electrons [22]. Petroleum phenols inhibit free radical processes most actively by reacting with short-lived radicals and thus preventing the further chain reaction [23]. Inhibitors can form reaction chains by several pathways, but in all the cases the products formed from an AO molecule should be less active than the stable radicals present in PS.
The AO amount in crude oils varies depending on the extent of their catagenetic transformation from 0.02 (J1, lower Jurassic) to 0.28 mol/kg (P–C, Permian–Carboniferous). Also, the AO concentration tends to decrease with an increase in the oil stratum depth [24]. The maximal AO concentration was found in asphaltenes, from 0.6 to 0.8 mol/kg; in resins, it was somewhat lower, of the order of 0.4–0.6 mol/kg. The concentration of petroleum AOs was found to correlate with the concentration of heteroatomic compounds, primarily of phenols in which the oxygen-containing functional groups are directly bonded to the carbon atoms of the aromatic ring and are shielded by short-chain hydrocarbon substituents. Such structures reproduce the structure of synthetic antioxidants terminating radical chains by the linear mechanism [24]. The AOA of petroleum phenols, evaluated by the rate constant of the reaction with the peroxy radical, k7, is of the order of n × 104 L mol–1 s–1, which is close to AOA of synthetic sterically encumbered phenols [25].
The studies performed suggest that resins and asphaltenes can be efficiently used as cheap stabilizers for plastics and rubbers [26]. However, technological attempts to introduce asphaltenes into plastics failed because of instability of the composition, heterogeneous distribution in the bulk of the plastic, and color becoming black. In addition, the concentration of AOs in asphatlenes is 10 and more times lower than in synthetic AOs because of a large amount of ballast substances exhibiting no activity in radical processes.
The Russkoe oilfield is one of the largest oilfields in Russia with respect to its reserves: 1.5 bln t of geological reserves and 410 mln t of recoverable reserves. The development of this oilfield is hindered by a number of factors such as high viscosity of the crude, complex geological section, lack of infrastructure, difficult development conditions, and severe climate (the temperature in the winter period varies from –30 to –50°С). The crude from this oilfield contains the minimal amount of light fractions and virtually no alkanes but has high content of low-index distillate oil fractions. To successfully recover the crude, particular attention is paid to trials of new highly efficient technologies, followed by their full-scale commercial implementation. Therefore, in this stage it is necessary to continue the research aimed at the development of novel high-tech equipment and complex technologies allowing reduction of the expenditures for the extraction, transportation, and refining of the crude oil.
In this study, we examined the effect that the wave treatment (namely, low-frequency acoustic waves and constant and alternating electromagnetic fields) exerts on the composition and antioxidant activity of fractions of asphaltenes, benzene-eluted resins, and benzene–alcohol-eluted resins separated from heavy high-viscosity crude of the Russkoe oilfield.
EXPERIMENTAL
Chemicals. In our study, we used n-hexane (chemically pure grade, EKOS-1, Russia), toluene (analytically pure grade, EKOS-1), ethanol (chemically pure grade, Vekton, Russia), ASKG silica gel (technical grade, Vekton), cumene (98%, Sigma–Aldrich C87657-1L), and azobis(isobutyronitrile) (AIBN) initiator (99%, analytically pure grade, Ekotek, Russia). n-Hexane, toluene, and ethanol were used without preliminary purification. ASKG silica gel was purified and dried according to GOST (State Standard) 11851-2008 (Crude Oil. Methods for Determining Waxes, Moscow: Standartinform, 2018). The pretreatment of cumene and AIBN is described in detail in [27].
Crude oil from the Russkoe oilfield belongs to the aromatic-naphthenic type. It is heavy (ρ20 870 kg/m3) and highly viscous (viscosity μ20 50 mPa s) and has low sulfur content (S = 0.39). It is low-wax (wax (paraffin hydrocarbon, PH) content 0.4 wt %), and, with respect to the content of resins and asphaltenes (ASPs), belongs to the resinous type (content of asphaltene–resin fraction from 10 to 20 wt %). The crude oil has relatively high content of nitrogen and oxygen (Table 1).
The crude oil treatment with constant electromagnetic field (ET1) was performed at room temperature with a permanent electromagnet with the magnetic induction of the order of 2 T for 2 h. The crude oil treatment with alternating electromagnetic field (ET2) was performed using a laboratory installation developed and fabricated at the Institute of Petroleum Chemistry, Siberian Branch, Russian Academy of Sciences. This installation can operate in a wide frequency range from 250 to 1200 Hz at a voltage from 10 to 17 kV. The following optimum ET2 parameters were chosen experimentally for the crude oil sample under consideration: voltage Н = 10 kV; frequency f = 900 Hz; optimum treatment time 15 min; room temperature.
For the low-frequency acoustic treatment of the crude oil, we used a laboratory analog of an industrial installation whose vibration system consists of a vibrating element (activator), elastic elements, and motor part immersed in the medium being treated [28]. The energy required for the process is transferred with an electromagnetic field to mobile elements of the installation, subjecting the whole volume of the liquid medium not only to acoustic and magnetic treatment, but also to intense stirring with high shear rates. The low-frequency acoustic treatment (LAT) leads to the break of the PDS structure and to a decrease in the viscosity and congealing point at lower power consumption than in thermal technologies [29]. The crude oil sample was treated in the stationary mode at room temperature and industrial frequency f = 50 Hz. The limiting magnetic field intensity H in the air gap of the device was 2×106 А/m, the installation power was 30 W, the sample volume was 0.3 dm3, and the treatment time was 20 min.
From the crude oil before and after the wave treatment, we separated by adsorption chromatography the fractions of asphaltenes and of benzene- and alcohol–benzene-eluted resins. Asphaltenes (ASPs) were precipitated with n-hexane. Benzene-eluted (BRs) and alcohol–benzene-eluted resins (ABRs) were separated by column liquid adsorption chromatography on ASKG silica gel using toluene and ethanol–toluene (1 : 1) as eluents, respectively. Paraffin hydrocarbons were separated according to GOST (State Standard) 11851-2008. In pretreatment of the crude oil sample, the content of mechanical impurities was determined according to GOST 6370-2018 (Crude Oil. Petroleum Products and Additives. Method for Determining Mechanical Impurities). The volume fraction of water in the crude was determined according to GOST 2477-2014 (Crude Oil and Petroleum Products. Method for Determining the Water Content). In further studies, we used crude oil samples containing no aqueous phase.
The relative content of structural fragments in ASPs was calculated from the IR spectra. The spectra were recorded with a Nicolet 5700 FTIR spectrometer using KBr plates in 1 : 300 ratio in the range 4000–400 cm–1. OMNIC 7.2 software (Thermo Nicolet Corporation) was used for processing the spectra and determining the optical density.
The antioxidant activity was studied with a custom-made MKDP-2 microcalorimeter fabricated at the Institute of Petroleum Chemistry, Siberian Branch, Russian Academy of Sciences [30]. Microcalorimetry is a kinetic method; it is based on recording the heat released in the model reaction of initiated oxidation of cumene (isopropylbenzene) in the presence of a sample containing АОs. The measurement procedure is described in detail elsewhere [27]. The device records the experimental curve of the heat release in the model reaction of the initiated oxidation of cumene. The induction period τ is determined from this curve.
The heat release power W (J/s) is related to the oxidation rate wоx as follows (1):
where ΔH is the enthalpy of the reaction, equal to 111±3 kJ/mol, and V is the cumene volume, L.
If there is a pronounced induction period τ, the initial antioxidant concentration [АО]0 in the natural sample can be calculated as follows (2):
where f is the stoichiometric coefficient of the inhibition (assumed to be equal to 2.0 for petroleum structures); n, number of functional groups (assumed to be equal to 1.0 for petroleum structures); [АО]0, initial antioxidant concentration in the sample, mol/kg; τ, induction period, s; and wi, initiation rate, mol L–1 s–1.
Examples of the heat release curves for the model reaction of the initiated cumene oxidation in the presence of alcohol–benzene-eluted resins are given in Fig. 1.
Heat release curves for the model reaction of the initiated cumene oxidation in the presence of alcohol–benzene-eluted resins separated from the (1) initial crude oil (С = 1.3 g/L) and (2) crude oil after ET2 (С = 1.0 g/L). Initiator: azobis(isobutyronitrile) (С in cumene 1 g/L); T = 60°C; wi = 6.8 × 10–8 mol L–1 s–1.
If there was no pronounced induction period τ in the experimental heat release curve for the oxidation in the presence of the test sample, the inhibiting activity was evaluated by a decrease in the final oxidation rate wоx. The uncertainty of the procedure was determined by the accuracy of measuring the induction period and the oxidation rate after the end of the induction period. For natural objects, it is ~10%.
Experiments on studying АОА of the crude oil and separated resins and asphaltenes were performed at 60°С in the presence of an oxidation initiator, azobis(isobutyronitrile) (AIBN) (concentration in cumene 1 g/L). The initiation rate wi for AIBN was 6.8×10–8 mol L–1 s–1 [31]. The concentrations of the samples were chosen taking into account the results of previous studies [19]. They were 1.5–2.0 g/L for the crude oil, 0.2 g/L for asphaltenes, and 0.2–1.0 g/L for the resin fractions. In the course of the experiment, the weighed sample was placed in the microcalorimeter cell charged with 2 mL of cumene, and the mixture was thermostated for 60 min at 60°С. After that, the initiator was added. Initially, an endothermic effect from the dissolution of AIBN was recorded, after which the curve reached the maximum of the heat release power, corresponding to the steady-state course of the process. While the sample inhibits the oxidation, the heat release is insignificant. After the AO in the sample is completely consumed, the experimental curve flattens out with the maximal heat release level. If the petroleum components contain weak AOs, the experimental curve demonstrates a slow increase in the heat release power without pronounced induction period.
RESULTS AND DISCUSSION
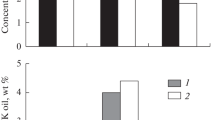
From the Russkoe oilfield crude before and after various kinds of wave treatment, we separated the fractions of asphaltenes and of benzene- and alcohol–benzene-eluted resins (Fig. 2).
Irrespective of the kind of the wave treatment, the content of the ASP fraction decreases to a similar extent, by a factor of more than 3.5. For resinous components, this trend is preserved, but after the acoustic and electromagnetic treatment (ET1 and ET2) the BR amount decreased by 26.0, 29.5, and 11.5 wt %, and the ABR amount, by 20.3, 17.4, and 18.8 wt %, respectively. This fact can be attributed to the release of condensed aromatic hydrocarbons and linear and branched hydrocarbons occluded in CSUs into the PS liquid hydrocarbon medium.
The effect of the wave treatment on the component composition of the asphaltene fraction was studied by IR spectroscopy. Figure 3 shows the IR spectra of asphaltenes isolated from the crude oil before and after LAT.
The spectra of ASP samples contain virtually all the characteristic absorption bands of the main functional groups of polyatomic organic molecules. The presence of aromatic rings in ASPs with different numbers of substituents is confirmed by the bands at 780, 794, 870, 1500, and 1610 cm–1, and the presence of sulfoxide groups, by the band at 1030 cm–1. The bands at 2920, 2850, 1456, and 1380 cm–1 are characteristic of alkyl groups.
To determine the relative content of structural fragments in ASPs, from the optical densities of the corresponding absorption bands we calculated the spectral coefficients of aromaticity С1 (А1600/А720), oxidation С2 (А1710/А1460), branching С3 (А1380/А1460), aliphaticity С4 ((А720 + А1380)/А1600), and sulfur content С5 (А1030/А1460) (Table 2). In addition, we determined the spectral coefficients reflecting the ratio of substituted bi- and tricyclic aromatic structures to the total content of aromatic fragments (А870/А1600) and the ratio of the sum of the aliphatic fragments (СН2 + СН3) to aromatic structures (А1460/А1600) [32].
The acoustic treatment of the crude oil leads to a decrease in the aromaticity (C1) of the isolated asphaltenes, whereas after the action of electromagnetic fields this parameter increases. The decrease in the aliphaticity (С4) is observed only after the treatment with alternating electromagnetic field (ET2). The coefficients of the oxidation (С2), branching (С3), and sulfur content (С5) of the ASPs change insignificantly after the wave treatment of the crude oil.
Comparison of the relative content of substituted arenes (А870/А1600) in the asphaltene samples shows that ASPs from the crude oil after ET2 contain a smaller amount of polycondensed aromatic structures and increased amount of monocyclic aromatic hydrocarbons, which are natural solvents for the polycondensed aromatic structures, enhancing the aggregative stability of resin–asphaltene aggregates.
Thus, the asphaltenes separated from the crude oil after various kinds of wave treatment only slightly differ in the content of aromatic and aliphatic structures, sulfoxides, and oxygen-containing compounds. It can be stated that the main processes in the PS after the action of external factors occur via rearrangement (aggregation and/or disaggregation) both inside CSUs and between them. This assumption, however, disagrees with the conclusions made by Tao and Xu [4], who believe that asphaltenes play the main role in the structure formation and apparently underestimate the role of resinous components and polycondensed aromatic structures. For example, we have already shown in previous studies [17, 19, 21] that application of a constant magnetic field leads to the breakdown of the existing PS structure through cleavage of weak hydrogen bonds and to the formation of additional reaction sites. When a magnetic field acts on a PS, both asphaltenes and resins exhibiting paramagnetic and diamagnetic properties show high activity. Depending on the crude oil type, its initial parameters are partially restored in 4–48 h, and the period of the complete restoration of the paramagnetic and antioxidant properties coincides with the time in which the rheological characteristics of the crude oil are restored.
To evaluate specific features of radical processes that occur in the crude oil and in RACs separated from it before and after the wave treatment of the oil, we examined the antioxidant activity and calculated the following parameters: induction period τ, oxidation rate wоx, and AO amount (Table 3). The AO content of the crude oil before the treatment is 0.048∙mol/kg, and the oxidation rate wоx is 10 times lower than that in the blank experiment, which suggests that the oil contains free radicals inhibiting the oxidation.
Irrespective of the kind of the action, the oxidation rate wоx in the model reaction in the presence of the crude oil sample decreases by a factor of 3.3 after LAT and ET2 and by a factor of 8 after ET1; i.e., AOA is enhanced. The AO amount increases from 6.3 to 16.7% owing to recombination of free radicals and formation of new molecular complexes (CSUs) in the PDS structure.
Analysis of the АО distribution between fractions has shown that the RAC separation is accompanied by the release of new reaction sites and formation of a larger amount of АОs compared to the initial crude oil (Table 4). For example, the oxidation rate wоx in the presence of the isolated asphaltene fraction and the AO amount in this fraction are 7 times higher compared to the untreated crude. An additional increase in wоx after the wave treatment was 5.4–13.4%, but after the acoustic treatment the AO amount increased by a factor of 1.5, which agrees with the IR data that the content of aromatic structures in ASPs decreases after LAT. It is known that the erosion of the CSU shell formed by low-polarity hydrocarbons of various structures, caused by such treatment, leads to a decrease in the PS viscosity and congealing point [29]. The electromagnetic treatment does not cause significant changes in the oxidation rate wоx and AO content because of the high polarity of the associated asphaltene structures in the crude oil sample [17, 18].
As seen from Table 4, AOA of the resinous components is considerably lower than that of asphaltenes because of lower content of paramagnetic structures and free radicals in the resins. The oxidation rate wоx with samples of neutral benzene-eluted and polar alcohol–benzene-eluted resins before the wave treatment is lower than that observed with ASPs by a factor of 3.3 and 1.9, and the АО content is lower by 23.5 and 41%, respectively. On the contrary, the induction period τ is considerably longer than that observed with asphaltenes. The wave treatment affects the reactivity of the resins differently. After the acoustic treatment of the crude oil, wоx with ABRs increases by 45%; after the treatment with the constant electromagnetic field, by 55%; and after the action of the alternating electromagnetic field, by 79%. The neutral resins after the treatment are characterized by a still greater increase in wоx: by factors of 2.0, 2.6, and 2.1, respectively. The quantitative increase in АО is the most significant for BRs: about 40% after any kind of treatment.
CONCLUSION
After subjecting heavy high-viscosity crude oil from the Russkoe oilfield to one of the kinds of the wave treatment (low-frequency acoustic field, constant or alternating electromagnetic field), the amount of the separated asphaltenes decreases by a factor of 3.5; that of benzene-eluted resins, by 11–30%; and that of alcohol–benzene-eluted resins, by up to 20%. This may be due to the breakdown of aggregates of complex structural units by the wave treatment with the release of liquid hydrocarbons of various structures, occluded in the molecular complexes, in the dispersion medium.
The action of physical fields leads to the formation of additional reaction sites and of a large amount of antioxidants in the existing petroleum-containing system owing to cleavage of weak hydrogen (or dispersion) bonds.
Changes in the kinetic parameters of oxidation inhibitors present in petroleum under the action of physical field suggest structural transformations involving changes in the size and activity of associative complexes of the petroleum system as a whole. In addition, the antioxidant activity of the separated resins is considerably lower than that of asphaltenes because of lower content of free radicals in the resin fraction.
REFERENCES
Du, E., Zhao, Q., Xiao, Y., Cai, L., and Tao, R., Fuel, 2018, vol. 220, pp. 358–362. https://doi.org/10.1016/j.fuel.2018.01.098
Li, H., Wang, X., Ma, C., Lu, Y., Han, S., Chen, C., and Zhang, J., Fuel, 2019, vol. 253, no. 1, pp. 647–661. https://doi.org/10.1016/j.fuel.2019.05.001
Taheri-Shakib, J., Shekarifard, A., and Naderi, H., JPSE, 2018, vol. 161, pp. 530–540. https://doi.org/10.1016/j.petrol.2017.12.012
Tao, R. and Xu, X., Energy Fuels, 2006, vol. 20, no. 5, pp. 2046–2051. https://doi.org/10.1021/ef060072x
Evdokimov, I.N. and Kornishin, K.A., Energy Fuels, 2009, vol. 23, no. 8, pp. 4016–4020. https://doi.org/10.1021/EF900296E
Goncalves, J.L., Bombard, A.J.F., Soares, A., Carvalho, D., and Nascimento, R., Energy Fuels, 2011, vol. 25, no. 8, pp. 3537–3543. https://doi.org/10.1021/ef101740b
Kulkarni, A.D. and Wani, K.S., IJSSBT, 2013, vol. 2, no. 1, pp. 36–39.
Mansoori, G.A., Khalaf, M.H., and Yong, C.W., JPSE, 2019, vol. 176, no. 8, pp. 926–933. https://doi.org/10.1016/j.petrol.2019.01.059
Taheri-Shakib, J., Shekarifard, A., and Naderi, H., J. Anal. Appl. Pyrol., 2018, vol. 129, pp. 171–180. https://doi.org/10.1016/J.JAAP.2017.11.015
Gao, J., Wu, P., Li, Ch., Xu, D., and Wang, X., Energies, 2023, vol. 16, p. 79. https://doi.org/10.3390/en16010079
Loskutova, Yu.V. and Yudina, N.V., Chem. Sustain. Develop., 2020, vol. 28, pp. 256–262. https://doi.org/10.15372/CSD2020228
Shang, H., Yue, Y., Zhang, J., Wang, J., Shi, Q., Zhang, W., Liu, L., and Omar, S., Fuel Process. Technol., 2018, vol. 170, pp. 44–52. https://doi.org/10.1016/j.fuproc.2017.10.021
Zhang, S., Sun, X., Yan, S., Liu, C., Miao, X., and Zhao, K., Phys. Fluids, 2022, vol. 34, ID 127122. https://doi.org/10.1063/5.0130925
Syunyaev, R.Z., Safieva, R.Z., and Safin, R.R., JPSE, 2000, vol. 26, nos. 1–4, pp. 31–39. https://doi.org/10.1016/S0920-4105(00)00018-8
Tung, N.P., Vuong, N.V., Long, B.Q., Vinh, N.Q., Hung, P.V., Hue, V.T., and Hoe, L.D., Soc. Petrol. Eng., 2001, pp. 17–19. https://doi.org/10.2118/68749-MS
Zlobin, A.A., Bull. PNRPU, Geol. Oil Gas Eng. Mining, 2017, vol. 16, no. 1, pp. 49–63. https://doi.org/10.15593/2224-9923/2017.1.6
Loskutova, Yu.V., Yudina, N.V., and Pisareva, S.I., Petrol. Chem., 2008, vol. 48, no. 1, pp. 51–55. https://doi.org/10.1134/S0965544108010106
Burlakova, E.B., Biooxidants: Yesterday, Today, and Tomorrow, Biologicheskaya kinetika: Sbornik obzornykh statei (Biological Kinetics: Coll. of Review Papers), Moscow, 2005, vol. 2, pp. 10–45.
Loskutova, Yu.V., Sizova, N.V., Yudina, N.V., and Petrenko, T.V., Petrol. Chem., 2005, vol. 45, no. 2, pp. 126–130.
Loskutova, Yu.V. and Yudina, N.V., Chem. Sustain. Develop., 2020, vol. 28, pp. 180–185. https://doi.org/10.15372/CSD2020218
Loskutova, Yu.V., Asphaltenes: Characterization, Properties and Applications, Ser.: Chemical Engineering Methods and Technology, Duncan, J.A., Ed., Nova Science, 2010, ID 121144.
Emanuel, N.M. and Lyaskovskaya, Yu.N., The Inhibition of Fat Oxidation Processes [Print Replica], Pergamon, 2013.
Tanaseychuk, B.S., Burtasov, A.A., and Pryanichnikova, M.K., Integr. Educ., 2015, vol. 19, no. 2, pp. 92–99. https://doi.org/10.15507/Inted.079.019.201502.092
Stakhina, L.D., Pisareva, S.I., Savinykh, Yu.V., and Sidorenko, A.A., Izv. Sib. Otdel. Akad. Nauk SSSR, Ser. Khim., 1988, no. 19, issue 6, pp. 131–135.
Halliwell, B., Free Radical Res., 1999, vol. 31, pp. 261–272.
Akhmedbekova, S.F., Khim. Tekhnol. Topl. Masel, 2012, no. 2, pp. 43–47.
Velikov, A.A., Karpitskii, V.I., and Sizova, N.V., Kinet. Katal., 1988, vol. 29, no. 2, pp. 321–325.
Daneker, V.A., Raschet i konstruirovanie elektromagnitnykh preobrazovatelei dlya aktivatsii zhidkikh sistem: Uchebno-metodicheskoe posobie (Calculation and Designing of Electromagnetic Converters for Activation of Liquid Systems: Methodical Tutorial), Tomsk: Tomsk. Politekh. Univ., 2018.
Loskutova, Yu.V., Prozorova, I.V., Yudina, N.V., and Rikkonen, S.V., Colloid J., 2005, vol. 67, no. 5, pp. 602–605.
Velikov, A.A. and Vichutinskii, A.A., USSR Inventor’s Certificate no. 1437696, Byull. Izobret., 1988, no. 42, p. 171.
Sizova, N.V., Chem. Sustain. Develop., 2023, vol. 31, no. 3, pp. 331–336. https://doi.org/10.15372/CSD2023474
Ivanova, L.V., Safieva, R.Z., and Koshelev, V.N., Vestn. Bashk. Univ., 2008, vol. 13, no. 4, pp. 869–874.
Funding
The study was performed within the framework of the government assignment for the Institute of Petroleum Chemistry, Siberian Branch, Russian Academy of Sciences and was financially supported by the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Loskutova, Y.V., Sizova, N.V. & Yudina, N.V. Effect of Wave Treatment on the Antioxidant Activity of Resins and Asphaltenes of Heavy High-Viscosity Crude Oil. Pet. Chem. 64, 548–556 (2024). https://doi.org/10.1134/S0965544124040017
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544124040017