Abstract
Biorefineries have been widely studied during the last years and proposed as the best option to transform biomass systems into value-added products. Different methodologies have been proposed for the design of biorefineries: knowledge-based approach, early-stage approach, and superstructures, among others, which have been coupled to process improvement schemes as pinch analysis and process intensification. Regarding process design, it is necessary to ensure that the most advanced concepts are applied on the design to ensure efficient processes that direct to more sustainable processes. The processes that are currently being designed as biorefineries in many cases cannot be considered as completely efficient, because they are generally biased to designs based only in typical methods of process engineering. It is still necessary to implement further design strategies focused on increasing the efficiency and decreasing energy consumption. Multiple methods have been used for the design and improvement of chemical/biotechnological processes. Among these, shortcut methods, especially thermodynamic-topological analysis, are highlighted as a useful tool in the process engineering stage of a given process and might be useful to decrease energy consumption and to increase the efficiency since the design stage. This work proposes a design strategy for biorefineries using a shortcut method as the thermodynamic-topological analysis. For this, a biorefinery based on cocoyam, which is a rural raw material with a high potential for the obtainment of added-value products, was used as a case study. The chosen products were ethanol, lactic acid, starch, and feed additive. The biorefinery was simulated in Aspen Plus. Thermodynamic-topological analysis was implemented in the proposed biorefinery and both biorefineries (with and without the application of thermodynamic-topological analysis) were assessed in economic, environmental, efficiency, and energy-consumption terms. It was determined that the application of thermodynamic-topological analysis generated a more efficient process. After the validation of the results, it was possible to establish a design strategy for biorefineries complementing the knowledge-based approach and based on thermodynamic-topological analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
During the last decade, biorefineries have been widely studied as one of the research keystones to transform the different biomass systems into value-added products [1]. Different methodologies have been proposed for the design of biorefineries: knowledge-based approach, early-stage approach, and superstructures, among others [2]. Since the design stage of biorefineries, it is necessary to ensure that the most advanced concepts are applied on the design to ensure efficient processes that direct to more sustainable processes. In this specific context, there are some problems, namely that processes being designed as biorefineries in many cases cannot be considered as completely efficient, because they are generally biased to designs based only in typical methods of process engineering. Additionally, despite of the creation of specific methods for biorefineries, i.e., the knowledge-based approach, it is still necessary to implement further design strategies focused on increasing the efficiency and decreasing energy consumption.
Improving energy efficiency becomes a topic of great transcendence for decreasing the environmental burden generated by the industry. Various stages and equipment require energy to perform within a process, regardless if it is heat, power or electricity. According to different authors [3–7], as a general perspective the process stage that consumes the most amount of energy is the separation/purification stage. This is because the real conversion and selectivity of most processes are not too high and mixtures entering the purification stage require a lot of processing to achieve the desired purity. Typically, as the purity increases, the cost increases too.
There are several methods for calculating the separation of multicomponent mixtures. Some examples of these methods are phase equilibrium and thermodynamic modeling, distribution coefficients and relative volatilities theory, the McCabe–Thiele and Savarit–Ponchon methods for binary mixtures, the properties and predictive methods of azeotropy, equilibrium and no equilibrium models with their respective MESH equation, among others. Despite the research performed on the conceptual design of multicomponent purification processes, the specification is still based on approximate methods [8]. Due to this, some shortcut methods have been proposed, both analytical and graphical, aiming to describe the behavior of multicomponent mixtures in multiple configurations of distillation columns.
One of the most practical shortcut methods is based on the graphic study of integral distillation trajectories under condition of infinite or finite reflux and in the differential distillation theory. It is applicable to mixtures of any number of components. The foundations of this method take as base the first description of distillation trajectories for ternary systems [9] and the posterior works of distillation paths from the chemical and physical equilibrium [10–14]. Serafimov and his research group directed their focus towards distillation schemes with total efficiency, i.e., under infinite reflux and number of stages [15, 16]. When the analysis of the trajectories behavior of a distillation process is performed under such conditions, it is commonly called thermodynamic-topological analysis [17]. The information that must be provided for this analysis is the boiling point of pure components and azeotropes, molar composition, composition of the mixture, solubility or liquid–liquid equilibria if present [13]. This information is very simple, making this methodology a very easy step for the establishment of the separation and purification zone of a given process.
Many methods have been used for the design and improvement of chemical/biotechnological processes. Among these, shortcut methods, especially thermodynamic-topological analysis, are highlighted as a useful tool in the process engineering stage of a given process and might be useful to decrease energy consumption and to increase the efficiency since the design stage. This work proposes a design strategy for biorefineries using a shortcut method as the thermodynamic-topological analysis. For this, a biorefinery based on cocoyam, with high potential for added-value products, was used as a case study. The chosen products were ethanol, lactic acid, starch, and feed additive. The biorefinery was simulated in Aspen Plus. Thermodynamic-topological analysis was implemented in the proposed biorefinery and both biorefineries (with and without the application of thermodynamic-topological analysis) were assessed in economic, environmental, efficiency and energy-consumption terms. It was determined that the application of thermodynamic-topological analysis generated a more efficient process. After the validation of the results, it was possible to establish a design strategy for biorefineries complementing the knowledge-based approach and based on thermodynamic-topological analysis.
MATERIALS AND METHODS
This work proposes a design strategy for biorefineries that complements the knowledge-based approach, widely used in the design of biorefineries [18–22], with the thermodynamic-topological analysis, aiming to increase the efficiency since the design stage. For this reason, the first section of this methodology will describe the steps for the implementation of thermodynamic-topological analysis. A case study using a rural raw material (cocoyam) with a high potential for the obtainment of added-value products was proposed. For this reason, the second section of the methodology describes the proposed biorefinery and contextualizes the raw material. Finally, the last section establishes the definition of efficiency used to evaluate both biorefineries (with and without the application of thermodynamic-topological analysis) and describes the indicators used for the techno-economic, environmental and energy assessment.
Implementation of Thermodynamic-Topological Analysis (TTA)
This section will address the implementation of TTA in the design of different stages of a biorefinery. The application of TTA consists in visualizing the thermodynamic behavior of the mixture using ternary or quaternary diagrams. The information to be provided for this analysis is very simple (boiling point of pure components and azeotropes, molar composition, composition of the mixture, solubility or liquid-liquid equilibria if present) [13], making this methodology a very easy step for the establishment of the separation and purification zone of a given process. The implementation has certain steps that will be explained as follows.
Selection of the equipment to implement thermodynamic-topological analysis. The first stage requires the identification of the equipment of the biorefinery and to define the equipment that can be designed and analyzed with TTA. The main types of processes that can be designed are purification and simultaneous reaction–separation processes. Both vapor–liquid equilibrium and nonequilibrium processes can be represented with TTA. This methodology can be applied for different distillation schemes such as conventional distillation, distillation trains with pressure swing, azeotropic distillation, extractive distillation, reactive distillation, distillation with pervaporation and reactive extraction, extractive fermentation and membranes [23–26].
Analysis previous to the application of thermodynamic-topological analysis. In general terms, TTA consists first in determining the boiling points of the compounds, selecting a thermodynamic model that describes correctly the vapor and liquid phases of the mixture, identifying the presence of azeotropes and liquid–liquid equilibria, graphing the ternary/quaternary diagrams and locating the respective azeotropes and liquid–liquid equilibria if any. Then, the separation regions (or concentration simplex) are identified with the residue curves and the distillation trajectories. Finally, once built this graph, it is possible to establish the possible separation scheme, the composition of the phases that are separated and so on [13]. Now, these steps will be developed as follows.
Identification of components. The first step is to identify the components that are present in the inlet stream that will enter to the chosen equipment and those with higher composition. The next step is to look for the boiling temperature of each of the components, given that this property shows the ease of separation of each of them.
Selection of the thermodynamic model. After determining the components of the mixtures, the next step is very important and it is the selection of a thermodynamic model that fits to the intrinsic properties and conditions for the mixture. The selection of the thermodynamic model will determine the properties of pure components and mixtures and it is completely vital that these properties are being estimated in an appropriate manner. Additionally, there is a wide variety of thermodynamic models with specific conditions to be applied, advantages and disadvantages. There are two “main categories” to divide the methods. The first one requires the fugacity coefficients for the phases, which can be determined with equations of state. The second one requires activity coefficients to describe the behavior of the liquid phase and the fugacity coefficients, which can be determined with activity models. Each one of the models has specific conditions to be applied. Some are specific for high-pressure or low-pressure processes, whether the process has electrolytes, polymers, carboxylic acids, amines, etc., or depending on the type of industry (petrochemical, pharmaceuticals, metallurgy, etc.). This context implies to know very well the compounds conforming the mixture, the operating conditions of the process [27].
Equilibrium diagrams for binary/ternary mixtures. Once chosen the thermodynamic model, the following step is to perform and analyze the equilibrium diagrams for the binary mixtures. These diagrams will give information about the presence of azeotropes and liquid–liquid equilibrium for the different pairing of the components. The following step is to perform and analyze the equilibrium diagrams for the ternary mixtures. The ternary diagram is focused on showing the presence of liquid–liquid equilibrium and ternary azeotropes, if any.
Graphing the residue-curve map and identification of concentration simplex separation regions). The next step is to graph the residue curve map for each of the ternary mixtures. A residue curve shows the change in composition of a mixture during continuous evaporation at the vapor–liquid equilibrium. Residue curves are not dependent of the presence of a LL dome and show the behavior of the liquid phase in an evaporation, in equilibrium with the vapor phase. The next step is to identify the concentration simplex or separation regions. These are the global regions that are formed within the triangle, which according to the behavior of the residue curves, determine the tendencies of separation of a mixture. This means that according to the specific conditions of the mixture, it is possible to have multiple low, intermediate and high boiling compounds and therefore different regions at which a given compound of the mixture would be purified.
Application of thermodynamic-topological analysis: determination of design variables.Extractive fermentation. It is now possible applying the thermodynamic-topological analysis to determine certain specific variables, in this case for the extractive fermentation. The most important variable to be determined for this stage is the required amount of solvent. The components of interest for an extractive fermentation are the substrate, the product, the solvent and water (representing the fermentation broth) [28]. There are some elements that should be identified. Given that the substrate is a solid soluble in water, it can be identified the solubility boundary of the substrate, which determines the maximum concentration that the aqueous solution is able to dissolve. Generally, the concentrations of the substrate never reach this concentration. The second element is the initial substrate concentration of the fermentation. The third element is the final concentration of the product in the fermentation. Finally, a line between the final concentration of product and the edge of the solvent represent the balance line for the addition of solvent. Now, in the ternary diagram water–product–solvent, the objective is to determine the amount of solvent to be added. The aim of the extraction solvent is to obtain an organic phase with a product concentration higher than that of the fermentation.
Distillation. The design variables to be determined for this process are: composition and flows of the distillate and bottoms, composition profile inside the column and feed stage [29]. For this analysis it is necessary to retake the residue curve map for the mixture but to complement it with further specific information. Some elements can be identified. The first element is the composition of the feed that enters the column. It is necessary to draw a straight line that starts on the desired point to be obtained as distillate and that passes by the feed. This is considered as a formulated distillate. The objective in this case is to obtain the highest possible concentration of the product in the distillate. The next element that can be identified is the “external residue curve”. This is the most outer residue curve that starts on the formulated distillate and crosses the triangle to the high boiling compound. In some point, the external residue curve and the formulated distillate will cross each other. This is the maximum composition that can be achieved for the distillate. Now, the composition of both the distillate and the bottoms are known and can be read from the graph. The flows of the distillate and bottoms can be determined either by the mass balances with the calculated compositions or using the “lever rule” for the line comprehended between the point of the distillate and the bottoms.
Summary of the application of thermodynamic-topological analysis. In general terms, it is possible to determine through the thermodynamic-topological analysis the most important design variables for different types of processes, as a simultaneous reaction-extraction and a typical vapor-liquid equilibrium purification process, which can be used as design variables in a simulation stage. The application of this shortcut method for the design of specific stages of a process turned out to be very practical. The application could be pointed out through certain specific steps that were easily identified and can be summarized as shown in Fig. 1. These steps are easy to follow and calculate, without further complication other than having software that calculates the ternary diagrams and the residue curves, which can be performed in simulation software as Aspen Plus or by free-code programming as Matlab.
Once applied the thermodynamic-topological analysis to determine the design variables of the chosen equipment, the next step is to proceed to the simulation software Aspen Plus and use the design variables (required solvent, feed stage, distillate and bottoms composition and flows and profile composition) in the respective equipment that is being simulated. Once specified these values, the next step is to corroborate the results of the simulator for the respective equipment and calculate if this short-cut method offers more efficient results and performance than the current design method of the software.
Description of the Cocoyam-Based Biorefinery
The study case of this work is a biorefinery based on cocoyam, which is a rural raw material with a high potential for the obtainment of added-value products. The chosen products were ethanol, lactic acid, starch and feed additive. The biorefinery was simulated in the software Aspen Plus. The respective biorefinery and raw material will be detailed as follows.
Raw material.Cocoyam. Xanthosoma sagittifolium (L.) Schott, known as cocoyam, belongs to the family of Araceas, whose origin is in Central America and it is grown mostly in tropical regions. This plant has global importance as an energetic meal. The main producing countries in the world are Nigeria, China and Ghana with more than 1.3, 1.182, and 0.9 million tons per year, respectively [30, 31]. The leaves of the plant have a high content of protein, the stem is mainly composed of lignocellulosics and the tuber has a considerable amount of starch (around 23 wt %), which is similar to other starchy raw materials as cassava and potatoes. The current uses of the plant are either directed as animal feed given the protein content and, in some cases, it is used as starch source. However, few studies have been proposed for an integral use of the plant.
The authors of [32] reported the characterization for the three parts of the cocoyam and the share of each of the parts in the entire plant. The leaves, stem and tuber represent 2.04, 14.97 and 82.99 (wt %) of the plant in a wet basis, respectively. Regarding the physicochemical characterization of the parts of the plant, the leaves have a content (wt % in dry basis) of 29.96, 16.68, 7.44, 38.38 and 7.53, for cellulose, hemicellulose, lignin, extractives and ash. The stem has a content (wt % in dry basis) of 31.34, 15.44, 10.76, 37.17, and 5.28, for cellulose, hemicellulose, lignin, extractives and ash. The tuber has a content (wt % in dry basis) of 24.52, 8.90, 6.26, 33.24, 3.45 and 23.64, for cellulose, hemicellulose, lignin, extractives, ash and starch. For the methodology of characterization refer to the reported work [32].
Proposed biorefinery. The synthesis of the flowsheet of the process was carried out with process simulation tools. The main objective of this stage was to determine the mass and energy balances of the process. The simulation took as base the obtained experimental results and when necessary the technological processes were complemented with kinetic models [33–36]. With the mass and energy balances, it was possible to calculate the requirements for raw material, utilities and energy. The software Aspen Plus v8.6 (Aspen Technology, Inc., USA) was used for the simulation of the process. Matlab (MathWorks, USA) was used for the mathematical modeling of the concentration profiles with kinetic models. The properties used to simulate the components of biomass were taken from the NREL/MP-425-20685 “Development of an ASPEN PLUS Physical Property Database for Biofuels Components” [37].
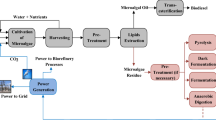
The proposed biorefinery consists in using the three parts of the plant, taking advantages of the components of the three platforms they have (protein, lignocellulosics and starch) to obtain an additive for animal feed, ethanol, lactic acid and starch. Figure 2 shows the basic scheme of the biorefinery with its products and by-products. The processing scale of the biorefinery is 2300 ton/day (26.62 kg/s).
The biorefinery will be explained as follows. First, the cocoyam is separated in its three parts. The tuber is submitted to a grinding and milling to extract the starch, which is purified and dried. The production process bases on the wet milling scheme for the production of starch from starchy raw materials [38, 39]. Starch is the product of this section. The remaining solids (non-extracted starch and lignocellulose) are mixed with the stem and enter to the pretreatment section for the production of fermentable sugars. In this second stage, the hemicellulose is removed through an acid hydrolysis using 2 wt % sulfuric acid at 121°C based on the kinetic expression reported by Jin et al. [33]. The produced xylose is detoxified and concentrated as a syrup and obtained as a by-product, together with gypsum. The cellulose and starch are submitted to two enzymatic hydrolysis, the first at 35°C to convert the cellulose into glucose and the second at 80°C to convert the starch into glucose, which is used as fermentable sugars. The enzymatic hydrolysis of cellulose was complemented with the kinetic expression reported by Morales-Rodríguez et al. [34].
The non-converted solids of this stage are mixed with the leaves and processed to obtain the additive for animal feed. The stream of fermentable sugars is split on a 50/50 ratio and used to ferment ethanol and lactic acid. The fermenting microorganism for ethanol production was Saccharomyces cerevisiae at 30°C. Considering the high concentration of glucose in the substrate stream, the concentration of ethanol in the fermentation exceeded the product inhibition limit of the microorganism (11–12% v/v of ethanol) [40, 41]. To avoid this inhibition, an extractive fermentation using n-dodecanol as solvent is proposed based on the research previously done by Quintero et al. [28]. The fermentation broth is mixed with n-dodecanol. The organic phase enters into an evaporator to recover the solvent, which is recycled. The aqueous phase enters to the purification step, consisting on a distillation column, in which ethanol is concentrated up to 50 wt %. Then, the distillate is mixed with the organic phase and enters the rectification column, in which ethanol is concentrated approximately to the azeotropic point (close to 96 wt %). Finally, ethanol is dehydrated until 99.6 wt % in a molecular sieve. The fermenting microorganism for lactic acid production was Lactobacillus casei at 37°C. The fermentation was complemented with the kinetic expression reported by Lin et al. [42]. The purification of the lactic acid comprises a precipitation, lactic acid recovery, evaporation, liquid–liquid extraction and distillation. Both products are purified and gypsum is obtained as a by-product of the production of lactic acid.
Determination of the Efficiency of the Biorefinery
Before doing any type of comparison of the efficiency of the biorefineries (with and without the application of thermodynamic-topological analysis), it is completely vital to give a comprehensive definition of what is going to be considered as “efficiency”, given the broad number of definitions that can be found in literature depending on the context. In very general terms, efficiency is to use the lowest amount of inputs to obtain the greatest amount of outputs. Now, as this work focuses on determining the efficiency of certain chemical processes, the inputs and the outputs to be considered must be necessarily related to the process. Therefore, the efficiency will be measured in technical, economic, environmental and energy terms.
The energy requirement of the process is calculated as the energy used in heat exchangers, boilers, pumps, compressors, mills, and reactors, among others. The economic assessment is performed to determine the Net Present Value (NPV) of the process, which is calculated based on the total cost of the process (capital cost, cost of utilities, raw materials and reactants, and operational costs) and the gross income of the products and by-products. A positive value of the indicator shows that the incomes are higher than the costs and the process has a positive performance. The cost of the equipment was calculated using the software Aspen Process Economic Analyzer v8.4. The capital depreciations, maintenance costs, labor costs, fixed charges, general and administrative costs and the plant overhead were calculated based on the percentages described for the economic assessment of chemical processes of [43].
For the economic assessment, the annual interest rate was 17% and an income tax of 25%. The period for the assessment is 10 years and the depreciation method was the straight line. The operation period was 8766 hours per year. Table 1 shows the cost of utilities, feedstock and reactants used in this stage. The commercial sales price of the products of the biorefinery used for the calculation of the NPV were 0.96, 1.54, 0.3, 0.4 and 1.00 USD/kg for ethanol, starch, lactic acid, gypsum, xylose syrup and animal feed, respectively. As there are no prices reported for an animal feed produced from the cocoyam, it was necessary to use the commercial sales price of an animal feed with similar composition.
For the environmental assessment, a methodology proposed by the United States Environmental Protection Agency was used. This software is called Waste Algorithm Reduction (WAR) [44] and it proposes three main impact categories: human toxicity, environmental toxicity and global warming. These main categories are subdivided into eight indexes, which are calculated depending on the components of the inlet and outlet streams. These eight indexes are used to weigh the Potential Environmental Impact (PEI). The software calculates the outlet impact (only considering the outlet streams) and the generated impact (the difference between the impact of outlet and inlet streams), for which a negative value indicates that the environmental impact of the given index has been decreased. The indexes considered in the calculation of the total PEI are human toxicity per ingestion (HTPI), human toxicity per dermal exposition or inhaling (HTPE), terrestrial toxicity potential (TTP), aquatic toxicity potential (ATP), global warming potential (GWP), ozone depletion potential (ODP), photochemical oxidation potential (PCOP) and acidification potential (AP).
For the calculation of the efficiency, regarding the economic performance the indicator to be used will be the Accumulated Net Present Value. Regarding the environmental performance, the indicator to be used will be the Total Potential Environmental Impact (PEI) Generated per Kilogram of Product. So far, these two indicators were already defined in the previous paragraphs. Regarding the technical performance, the indicator to be used will be the Productivity, defined as the kilograms of products obtained per kilogram of raw material. Finally, considering that the thermodynamic-topological analysis aims to be applied on the purification stage of the process and typically processes as distillation tend to be very energy-consuming, in terms of energy performance the indicator to be used will be the Energy Consumption, defined as the energy consumed per kilogram of raw material. Table 2 shows the remarks and specifications of the four variables that will be calculated to determine the efficiency.
Considering that the efficiency is going to be a factor to compare two biorefineries, the aim or the consideration of an efficient process with respect to other will be having a higher value of the Accumulated NPV at the end of the project lifetime, lower value of the Total PEI per kilogram of product, higher value of Productivity and a lower value of Energy Consumption.
RESULTS
Application of Thermodynamic-Topological Analysis
This section will address the application of the described steps for thermodynamic-topological analysis.
Selection of the equipment to implement thermodynamic-topological analysis. Having established the technological scheme, it is necessary to identify the equipment that conforms each of the stages of the process. This information is shown in Table 3. According to the equipment at each stage and the applications of thermodynamic-topological analysis, the stage with the highest number of equipment that can be designed with thermodynamic-topological analysis is the ethanol production stage. The equipment that will be designed using thermodynamic-topological analysis will be the extractive fermentation, the distillation column, and the rectification column. This three equipment show different technologies that can be designed with thermodynamic-topological analysis, namely a distillation and simultaneous reaction–extraction through liquid–liquid extraction coupled to fermentation.
Analysis previous to the application of thermodynamic-topological analysis. Once determined the equipment, it is necessary to identify the compounds in the inlet stream of the chosen equipment, their properties and so on, which will be done as follows.
Identification of components. For extractive fermentation, considering that it is a simultaneous reaction–separation process, it is necessary to know the amount of ethanol that is being produced and therefore both the current inlet and outlet flow must be known. For the distillation and rectification columns, given that they are only separation processes based on the vapor–liquid equilibrium, it is only necessary to know the components that go into the feed. For the extractive fermentation the components with the higher concentrations are glucose (substrate), ethanol (product), water (broth) and dodecanol (solvent). For the distillation column, which processes the aqueous phase that goes out of the decanter, the components with higher concentration are water, ethanol and dodecanol. For the rectification column, which processes the distillate of the distillation column mixed with the organic phase once the solvent is recovered, the components with higher concentration are water, ethanol and dodecanol. The extractive fermentation is the only case that will have four components.
Having identified the components, the next step is to look for the boiling temperature of each of the components, given that this property shows the ease of separation of each of the components. This information was determined from [45]. Glucose is a solid soluble in water. The boiling points of water, ethanol, glucose and dodecanol at 1 atm are 100, 78.37, 343.85 and 259°C, respectively. There are considerable differences in the boiling points of the three components, which is the first requirement to perform a vapor–liquid separation of each of the components to obtain them pure.
Selection of the thermodynamic model. After determining the components of the mixtures, the next step is very important and it is the selection of a thermodynamic model that fits to the intrinsic properties and conditions for the mixture. This biorefinery does not include electrolytes, operates below 10 bars and has the presence of two liquid phases. Due to this, the Non-Random Two Liquids (NRTL) model was chosen to describe the liquid phase. Other possible methods were the UNIQUAC and the UNIFAC-LL, which has special interaction parameters for LLE applications. However, NRTL model describes VLE and LLE of strongly non–ideal solutions of polar and non–polar compounds. The restriction is that no component should be close to its critical temperature and it requires binary parameters, which in this case were taken from the databank of the simulator. For the description of the vapor phase, the Hayden–O’Connell equation of state was used, because the process has the presence of carboxylic acids and when they are in vapor phase they tend to dimerize, and this equation of state considers this.
Equilibrium diagrams for binary/ternary mixtures. The following step is to perform and analyze the equilibrium diagrams for the binary/ternary mixtures. Figure 3 shows the respective vapor–liquid diagrams for the binary mixtures at 1 atm. The first three graphs (a, b and c) show the vapor–liquid equilibrium for glucose with the other three components, water, ethanol and dodecanol, respectively. Although glucose is a soluble solid, it can modify the boiling points of liquid mixtures. Figures 3d and 3e show the vapor–liquid equilibrium of water with ethanol and dodecanol, respectively. The water–ethanol vapor–liquid equilibrium shows an azeotrope and the water–dodecanol vapor–liquid equilibrium shows a two-phase liquid–liquid equilibrium. Finally, Fig. 3f shows the vapor–liquid equilibrium of ethanol and dodecanol, and there is no presence of azeotropes or liquid–liquid equilibrium. The water/ethanol azeotrope appears at a temperature of 78.15°C (minimum boiling point azeotrope) and at a mole fraction for water/ethanol of 0.1048/0.8952 (0.0438/0.9562 in mass fraction).
The ternary diagram is focused on showing the presence of liquid–liquid equilibrium and ternary azeotropes, if any. Therefore, the combinations of the components will be restricted to water–dodecanol–ethanol, given that water–dodecanol are the only components that generated a liquid–liquid equilibrium. Figure 4 shows the respective ternary diagrams. It can be observed that there are no ternary azeotropes. The liquid–liquid dome has a very favorable tendency, because the tie-lines have a negative slope and the border on the organic phase has a higher concentration of the compound of interest (ethanol) than the aqueous phase. The second element of the LL dome that favors these mixtures is that the side of the dome corresponding to the aqueous phase (right side) is very close to the right side of the triangle, implicating that the concentration of water is very high, without further presence of the solvent.
Graphing the residue-curve map and identification of concentration simplex (separation regions). The next step is to graph the residue curves map for each of the ternary mixtures. Figure 5a shows the residue curves for the water–ethanol–dodecanol mixture. It can be observed that the residue curves go from the water–ethanol binary azeotrope, given that it is a minimum temperature azeotrope with a boiling temperature lower than that of pure ethanol and therefore it is the low boiling component. The intermediate boiling components in this case are water and ethanol, and the high boiling component is dodecanol. This behavior implies that it is possible to obtain pure dodecanol, but water and ethanol require a type of process different to conventional evaporation methods (as distillation) to obtain them pure.
The next step is to identify the concentration simplex or separation regions. In this specific case, there is only one concentration region corresponding to all the interior of the triangle. This is because all the residue curves have the same tendency, the same low boiling compound and high boiling compound. Figure 5b shows the concentration simplex for the main components at 1 atm. However, as it was analyzed in the previous section with the residue curve map, the current mixture poses a problem for a purification process based on the vapor–liquid equilibrium, because the water–ethanol binary azeotrope determines the start point of the region. This implies that at a given pressure it is only possible to separate the azeotrope and dodecanol, but it is not possible to obtain either water or ethanol pure.
Application of thermodynamic-topological analysis: determination of design variables. After performing all the previous analysis, the next step is to apply specifically thermodynamic-topological analysis to the chosen equipment to determine certain design variables. This application will be performed in the following sections to the extractive fermentation and the distillation and rectification columns.
Extractive fermentation. The most important variable to be determined for this stage is the required amount of solvent. Regarding the extractive fermentation, this is the only stage with four components, so the first analysis will be the quaternary diagram of system. Figure 6 shows the quaternary diagram for glucose, water, ethanol and dodecanol. With respect to the analysis performed in the previous section for this mixture, it is necessary to consider other elements. The first element is the glucose solubility boundary in an aqueous solution (grey-colored triangle), which is approximately 0.909 in mass fraction [45]. The second element is the initial substrate concentration of the fermentation (point A in red), which specifically for this case is 0.46 in mass fraction. The third element is the final concentration of ethanol in the fermentation (point B in blue), which specifically for this case is 0.22 in mass fraction. Finally, the point in green represents the pure solvent. The line AB represents the fermentation trajectory and the line BC represents the addition of solvent.
Now, it is possible to move to the water–ethanol–dodecanol ternary diagram considering that all substrate is consumed. The objective is to determine the amount of solvent to be added. For this, it is necessary to determine the operation range, which is limited in this case by two conditions: the maximum substrate solubility and the ethanol inhibition concentration. Therefore, the ethanol in the aqueous phase must be below the inhibitory concentration (approximately 18 wt %) and the substrate initial concentration must be below the solubility limit.
The idea of the addition of solvent is to remove the most amount ethanol and that the organic phase has a higher concentration of ethanol that the initial concentration, so that after the recovery of the solvent, the concentration of ethanol is higher. Figure 7 shows an example of a chosen solvent amount and the respective concentration of ethanol obtained after the removal of the solvent. The red line shows the mass-balance line equivalent to the addition of the solvent. The initial point on the ethanol–water line shows the achieved concentration of ethanol in the fermentation without any amount of solvent. As the amount of solvent increases, the solvent line goes to the border of the liquid–liquid dome. A given flow of solvent will determine a specific point in the red line, represented by the mole fraction of dodecanol, water and ethanol. According to the liquid–liquid dome, only one tie-line (yellow line) will pass by this point and it will indicate the concentration of both phases that will be created when the mixture is allowed to decant (decanter). The organic phase is taken then to a stage of recovery of the solvent and the ethanol and water remain, which is represented with the black dotted line, and the final estimated concentration of ethanol is shown. This final concentration should be higher than the initial one.
The objective of the extraction solvent is to obtain an organic phase with an ethanol concentration higher than that of the fermentation. Based on this, the chosen flow of solvent is 1 kmol/h (0.278 mol/s), which is a value that allows obtaining approximately an ethanol mole fraction of 0.26 and a flow of 0.65 kmol/h (0.181 mol/s), which is more than 60% of the ethanol produced in the fermentation.
Distillation. The other two equipment chosen to be designed with topological thermodynamic analysis are the distillation and the rectification columns (both distillation columns). In terms of procedure, the application of TTA is the same, but the specific components and concentrations of the columns differ from each other. The design variables to be determined for this equipment are: composition and flows of the distillate and bottoms, composition profile inside the column and feed stage.
Distillation column. For the distillation column, which processes the aqueous phase that goes out of the decanter, the components with higher concentration are water, ethanol, and dodecanol. For this analysis it is necessary to retake the residue curve map for this mixture and complement it with further specific information. Figure 8 shows the respective distillation synthesis map, which shows the concentration simplex for this specific mixture (only one), and all the curves starting from the azeotrope and going to the high boiling component (dodecanol). Some elements can be identified in the figure. The first element is the composition of the feed that enters the column. The mole fraction of the feed is 0.93, 0.069 and 0.001 for water, ethanol and dodecanol, respectively. It can be observed that the composition of the feed is almost on the side of the triangle that represents a binary water–ethanol mixture. Therefore, as dodecanol composition can be almost despicable, the two components that will be aimed to be purified are water and ethanol.
It is necessary to draw a straight line that starts on the desired point to be obtained as distillate and that passes by the feed (formulated distillate). This line is shown as the red line. The objective in this case is to obtain the highest possible concentration of the ethanol in the distillate, which is the azeotrope, and the water in the bottoms. The next element that can be identified is the “external residue curve” (green colored line with an arrow). Given that the composition of the feed is almost on the water–ethanol side of the triangle, the formulated distillate will be also practically superposed to this side of the triangle. In some point, the external residue curve and the formulated distillate will cross each other. This is the maximum composition that can be achieved for the distillate and the bottoms will be on the corner of pure water. These points are shown respectively in the figure as blue circles with the words “distillate” and “bottoms”. Now, the composition of both the distillate and the bottoms are known and can be read from the figure. Table 4 shows the respective composition and flows of the distillate and bottoms.
For the calculation of other design variables, and to observe better how they are determined graphically, the previous figure will be framed only to the region of interest (Fig. 9). The following steps consist in calculating the composition profile of the liquid phase. To do so, it is fixed a number of stages for the column and the portion of the external residue curve that goes from the distillate point to the bottoms is divided in equidistant segments corresponding to the fixed number of stages. Each of the divisions will correspond to the composition of the liquid phase in equilibrium for the stages. TTA does not allow determining a number of stages of the column and it is necessary to do a sensitivity analysis to determine this number or appeal to other short-cut methods as the Fenske–Gilliland–Underwood or Winn–Underwood–Gilliland to calculate the minimum number of stages. In this case, the used number of stages is 20, given that the typical values for distillation columns of ethanol are between 18–25 stages [5]. Finally, the last variable that can be determined is the feed stage, a decision that is based in the composition of the feed and the profile composition. This stage corresponds to the segment that crosses the point at which it is located the feed, which is in this case is the stage 13. This is all the topological thermodynamic analysis corresponding for the distillation column.
Rectification column. The following step is to perform the same strategy and analysis performed in the previous section for the distillation column, but now for the specific conditions and compositions of the rectification. The rectification column processes the distillate of the distillation column mixed with the organic phase once the solvent is recovered. Figure 10 shows the respective distillation synthesis map, which shows the concentration simplex and some elements can be identified in the figure. The first element is the composition of the feed that enters into the column. The mole fraction of the feed stream is 0.632, 0.333 and 0.035 for water, ethanol and dodecanol, respectively. In this case, the composition of the feed is not over the side of the triangle that represents a binary water–ethanol mixture.
The following step is to draw the formulated distillate line starting in the ethanol–water azeotrope and that passes by the feed (red line). The next element is the “external residue curve” (green colored line with an arrow). The crossing point between the external residue curve and the formulated (maximum composition for the distillate) and bottoms are shown respectively in the figure as blue circles with the word “distillate” and “bottoms”. The composition of both the distillate and the bottoms are known and can be read from the figure. Table 5 shows the respective composition and flows of the distillate and the bottoms of the distillate and bottoms.
Figure 11 shows the framing of the region of interest for the calculation of the composition profile of the liquid phase. The number of stages fixed was 30 given that the typical values for rectification columns of ethanol are between 25–30 stages [5] and the external residue curve that goes from the distillate point to the bottoms is divided in equidistant segments corresponding to the fixed number of stages. Finally, the last variable that can be determined is the feed stage, which in this case is stage 15.
Determination of the Efficiency of the Biorefinery
After running the respective simulations and performing the respective calculations, the following step is performing the comparison with respect to the indicators previously mentioned. Considering that the ethanol production stage was the one chosen to be designed with thermodynamic-topological analysis, the Specific Productivity indicator will be calculated as the mass flow of ethanol with respect to the mass flow of raw material for the processing scale and the Energy Consumption indicator will be done only with respect to the equipment directly involved in the application of TTA. These are the decanter, the evaporator to recover the solvent, and the condenser and reboiler of the distillation and rectification columns.
Accumulated NPV. Regarding the Accumulated NPV, it was proceeded to calculate the costs and incomes of the cocoyam-based biorefinery after simulating with the design variables determined with the application of the TTA, and then to calculate the Accumulated NPV for the chosen processing scale of 2300 ton/day. It was determined that for the case with the application of TTA, the Accumulated NPV at the end of the 10-year period of lifetime or the project was 69.68 million USD, with a payback period of 4.46 years. The case without the application of TTA generated an Accumulated NPV of 63.30 million USD with a payback period of 4.67 years. The case with the application showed better performance in this aspect and this can be explained because as the requirement of utilities decreases (which is discussed in more detail in the later section of Energy Consumption), the costs of the process decrease, and the produced ethanol increases (discussed in more detail in the later section of Productivity). The increase in the Accumulated NPV for the case with the application of TTA is approximately 10.55%, with a decrease in the payback period of 0.21 years.
Environmental performance. Regarding the Environmental Performance, Table 6 shows the results obtained for the calculation of the Potential Environmental Impact leaving the system and generated by the system. It can be observed that there is a slight difference in the values obtained in both cases, favoring the case with the application of TTA. The total PEI leaving the system is smaller and the total PEI generated by the system has a bigger negative value. This implies that the streams leaving the system are less contaminant. This can be explained because as the purification process of ethanol becomes better, less ethanol goes in the outlet stream of the process and therefore the environmental impact decreases. In this regard, the decrease of the Environmental Impact for the case with the application of TTA is approximately 0.73%.
Productivity. Regarding Productivity, it was observed that with the application of TTA there was a slight increase in the flow of ethanol. This can be explained because the determination of the concentration profile and the estimates on the simulation gives a better indicator of the behavior that the mixture will have and therefore it is possible to purify more of the product. For the case with the application of TTA, the ethanol flow was 46.98 kg/h, while for the case without the application of TTA it was 45.57 kg/h. The Productivity indicator was 22.55 and 21.87 kg of ethanol per ton of cocoyam for the case with and without the application of thermodynamic-topological analysis, respectively. The increase in the Productivity was 3.09%, favoring the application of TTA.
Energy consumption. Regarding Energy Consumption, the energy consumption of each piece of equipment (decanter, evaporator, condensers and reboilers of the distillation and rectification columns) was determined. Energy was considered to be consumed despite if it is to remove or to supply energy in the form of heat. Table 7 shows the energy required for each piece of equipment and the share it represents. It can be observed that the decanter and the evaporator have the same energy requirement in both cases. In both cases, distillation and rectification are the processes that require the highest amount of energy, accounting for more than 80% of the share. However, regarding distillation and rectification, the application of TTA decreased the consumption of energy. Energy Consumption indicator has values of 0.585 and 0.592 MJ per kilogram of cocoyam, with and without the application of thermodynamic-topological analysis. This represents a decrease in the energy requirements of approximately 1.22% in one of the most energy-consuming stages of a process, as it is the distillation.
Final results. After performing the respective analysis and comparison of the aforementioned indicators, it was determined that the application of thermodynamic-topological analysis generated as outcome a process more efficient than that without its application. This result is supported by the fact that the process designed with the application of TTA proved having better performance in the four criteria used to define the efficiency in economic, environmental, technical and energy terms (Higher value of the Accumulated NPV at the end of the project lifetime, Lower value of the Total PEI per Kilogram of Product, Higher value of Productivity and Lower value of Energy Consumption).
STRATEGY BASED ON THERMODYNAMIC-TOPOLOGICAL ANALYSIS FOR THE DESIGN OF BIOREFINERIES
Once proven the efficiency of the designed biorefinery and validated that the application of thermodynamic-topological analysis in the design stage of a biorefinery will result in a more efficient process, it is possible to define a design strategy, which is done as follows. The final step is to propose a design strategy that includes the thermodynamic-topological analysis. The main structure will be based on the knowledge-based approach and on the design strategy proposed by Moncada et al. [1]. Figure 12 shows the scheme of the proposed design strategy for biorefineries based on thermodynamic-topological analysis. The strategy will be detailed as follows.
The first stage is the selection of the raw material. The second stage is hierarchy, applied to the feedstock and technologies. Regarding feedstocks, a given raw material will allow obtaining a certain type of products based on its composition. Regarding technologies, hierarchy focuses on the step that affects the most each process. The third stage is sequencing, which aims to establish a logical order and relation between technologies and products. This stage implies the selection of desired products, definition of a main product and by-products, the available technologies and the restrictions of product quality. It is very important to define clearly the purpose of the biorefinery.
The fourth stage is thermodynamic-topological analysis, which aims to design the reaction–separation and/or separation stages of the process. The fifth stage is integration, which aims to take advantage of all the mass and energy streams within the process. Mass integration, heat integration and process intensification are the main components of this stage. The sixth stage is the pre-feasibility analysis, which comprehends the assessment of the process in economic and environmental terms.
CONCLUSIONS
Thermodynamic-topological analysis proved to be a useful tool for the design of efficient processes. The efficiency of the processes designed with and without the application of thermodynamic-topological analysis was validated in technical, economic, environmental and energetic terms. Finally, after the validation of the results, it was possible to establish a design strategy for biorefineries, based on thermodynamic-topological analysis, aiming to obtain efficient biorefineries.
Regarding thermodynamic-topological analysis, it was shown that this is a useful tool for the design of efficient processes, considering efficiency under the technical, economic, environmental and energetic terms defined herein. The thermodynamic-topological analysis allowed determining the most important design variables for different types of processes, as a simultaneous reaction–extraction and a typical vapor–liquid equilibrium purification process, improving the performance of the designed reaction–separation and separation stages in the biorefinery, and with that the overall efficiency of the biorefinery. Compared to other design methods, this shortcut method does not require a lot of information and can be graphically designed with the use of ternary and quaternary diagrams. Finally, regarding the overall design of biorefineries, the proposed design strategy took as base the thermodynamic-topological analysis and complemented the current state-of-the-art for the design of biorefineries addressing the problem that the processes that are currently being designed as biorefineries in many cases cannot be considered as completely efficient.
REFERENCES
Moncada, J.B., Aristizábal, V.M., and Cardona, C.A., Design strategies for sustainable biorefineries, Biochem. Eng. J., 2016, vol. 116, p. 122.
Kamm, B., Kamm, M., Gruber, P., and Kromus, S., Biorefinery systems—An overview, Biorefineries— Industrial Processes and Products: Status Quo and Future Directions, Kamm, B., Gruber, P.R., and Kamm, M., Eds., New York: Wiley-VCH, 2010, p. 3–40.
Seider, W.D., Seader, J.D., and Lewin, D.R., Product & Process Design Principles, New York: Wiley, 2004.
Douglas, J.M., Conceptual Design of Chemical Processes, New York: McGraw-Hill, 1988.
Seader, D. and Henley, E.J., Separation Process Principles, New York: Wiley, 2006.
Kanegsberg, B. and Kanegsberg, E., Handbook for Critical Cleaning, Washington, DC: CRC, 2000.
Industrial Energy Efficiency, Washington, DC: DIANE, 1993.
Adiche, C. and Vogelpohl, A., Short-cut methods for the optimal design of simple and complex distillation columns, Chem. Eng. Res. Des., 2011, vol. 89, p. 1321.
Fah, S., Dampfdrucke ternarer gemische. II. Theoretischer teil, Z. Phys. Chem., 1901, vol. 36, p. 413.
Brzostowski, W. and Malanowski, S., Vapour-liquid equilibria in binary systems of pyridine bases, Bull. Acad. Pol. Sci., Ser. Sci., Chim., Geol. Geogr., 1959, vol. 7, p. 669.
Prigogine, I. and Henin, F., On the general theory of the approach to equilibrium. I. Interacting normal modes, J. Math. Phys., 1960, vol. 1, p. 349.
de Groot, S.R., Mazur, P., and King, A.L., Non-equilibrium thermodynamics, Am. J. Phys., 1963, vol. 31, p. 558.
Pisarenko, Y.A., Serafimov, L.A., Cardona, C.A., Efremov, D.L., and Shuwalov, A.S., Reactive distillation design: Analysis of the process statics, Rev. Chem. Eng., 2001, vol. 17, no. 4, pp. 253–327. https://doi.org/10.1515/REVCE.2001.17.4.253
Serafimov, L.A., Timofeev, V.S., Pisarenko, Y.A., and Solokhin, A.V., Tekhnologiya osnovnogo organicheskogo sinteza. Sovmeshchennye protsessy. Uchebnoe posobie dlya vuzov (Basic Organic Synthesis Technology. Combined Processes: A Textbook for Institutions of Higher Education), Moscow: Khimiya, 1993.
Serafimov, L.A., Petlyuk, F.B., and Kievskii, V.Y., Thermodynamic and topological analysis of phase diagrams of polyazeotropic mixtures. 1. Determination of distillation regions using a computer, J. Phys. Chem., 1975, vol. 49, p. 1834.
Serafimov, L.A., Petlyuk, F.B., and Kievskii, V.Y., Thermodynamic and topological analysis of phase diagrams of polyazeotropic mixtures. 2. Algorithm for construction of structural graphs for azeotropic ternary mixtures, J. Phys. Chem., 1975, vol. 49, p. 1836.
Zharov, V.T. and Serafimov, L.A., Fiziko-khimicheskie osnovy distillyatsii i rektifikatsii (Physicochemical Fundamentals of Distillation Processes), Leningrad: Khimiya, 1975.
Moncada, J., Cardona, C.A., and Rincón, L.E., Design and analysis of a second and third generation biorefinery: The case of castorbean and microalgae, Bioresour. Technol., 2015, vol. 198, p. 836.
Cardona Alzate, C.A., Solarte-Toro, J.C., and Peña, Á.G., Fermentation, thermochemical and catalytic processes in the transformation of biomass through efficient biorefineries, Catal. Today, 2018, vol. 302, p. 61.
Gutiérrez, L.F., Sánchez, Ó.J., and Cardona, C.A., Process integration possibilities for biodiesel production from palm oil using ethanol obtained from lignocellulosic residues of oil palm industry, Bioresour. Technol., 2009, vol. 100, p. 1227.
Daza Serna, L.V., Solarte Toro, J.C., Serna Loaiza, S., Chacón Perez, Y., and Cardona Alzate, C.A., Agricultural waste management through energy producing biorefineries: The Colombian case, Waste Biomass Valorization, 2016, vol. 7, p. 789.
Hernández, V., Romero-García, J.M., Dávila, J.A., Castro, E., and Cardona, C.A., Techno-economic and environmental assessment of an olive stone based biorefinery, Resour., Conserv. Recycl., 2014, vol. 92, p. 145.
Pisarenko, Y.A., Danilov, Yu.R., and Serafimov, L.A., Study of modes for the reactive distillation analysis of statics, Theor. Found. Chem. Eng., 1995, vol. 29, no. 6, p. 612.
Cardona, C.A., Sánchez, C.A., and Gutiérrez Mosquera, L.F., Análisis de la estática en procesos de destilación reactiva, in Destilación Reactiva: Análisis y Diseño Básico, Sede Manizales, Manizales: Universidad Nacional de Colombia, 2007, p. 375.
Peters, M., Glasser, D., Hildebrandt, D., and Kauchali, S., Membrane Process Design Using Residue Curve Maps, Hoboken, N.J.: Wiley, 2011.
Peters, M., Kauchali, S., Hildebrandt, D., and Glasser, D., Derivation and properties of membrane residue curve maps, Ind. Eng. Chem. Res., 2006, vol. 45, p. 9080.
Serafimov, L.A., Pisarenko, Y.A., and Kulov, N.N., Coupling chemical reaction with distillation: Thermodynamic analysis and practical applications, Chem. Eng. Sci., 1999, vol. 54, p. 1383. https://doi.org/10.1016/S0009-2509(99)00051-2
Gutiérrez, L.F., Sánchez, Ó.J., and Cardona, C.A., Analysis and design of extractive fermentation processes using a novel short-cut method, Ind. Eng. Chem. Res., 2013, vol. 52, p. 12915.
Quintero, J.A. and Cardona, C.A., Process simulation of fuel ethanol production from lignocellulosics using aspen plus, Ind. Eng. Chem. Res., 2011, vol. 50, p. 6205.
Roots, Tubers, Plantains and Bananas in Human Nutrition, Rome: Food and Agriculture Organization of the United Nations, 1998.
Onwueme, I.C. and Charles, W.B., Tropical Root and Tuber Crops: Production, Perspectives and Future Prospects, Rome: Food and Agriculture Organization of the United Nations, 1994.
Serna-Loaiza, S., Martínez, A., Pisarenko, Y., and Cardona-Alzate, C.A., Integral use of plants and their residues: The case of cocoyam (Xanthosoma sagittifolium) conversion through biorefineries at small scale, Environ. Sci. Pollut. Res., 2018, vol. 25, no. 36, pp. 35949–35959. https://doi.org/10.1007/s11356-018-2313-7
Jin, Q., Zhang, H., Yan, L., Qu, L., and Huang, W., Kinetic characterization for hemicellulose hydrolysis of corn stover in a dilute acid cycle spray flow through reactor at moderate conditions, Biomass Bioenergy, 2011, vol. 35, no. 10, p. 4158.
Morales-Rodriguez, R., Gernaey, K.V., Meyer, A.S., and Sin, G., A mathematical model for simultaneous saccharification and co-fermentation (SSCF) of C6 and C5 sugars, Chin. J. Chem. Eng., 2011, vol. 19, p. 185.
Leksawasdi, N., Joachimsthal, E., and Rogers, P., Mathematical modelling of ethanol production from glucose/xylose mixtures by recombinant Zymomonas mobilis, Biotechnol. Lett., 2001, vol. 23, no. 13, p. 1087.
Min, D.-J., Choi, K.H., Chang, Y.K., and Kim, J.-H., Effect of operating parameters on precipitation for recovery of lactic acid from calcium lactate fermentation broth, Korean J. Chem. Eng., 2011, vol. 28, p. 1969.
Wooley, R.J. and Putsche, V., Development of an ASPEN PLUS Physical Property Database for Biofuels Components, Golden, Colo.: National Renewable Energy Laboratory, 1996.
Aristizábal, J. and Sánchez, T., Guía técnica para la producción y análisis de almidón de yuca, Rome: Food and Agriculture Organization of the United Nations, 2007.
Owusu-Darko, P.G., Paterson, A., and Omenyo, E.L., Cocoyam (corms and cormels)—An underexploited food and feed resource, J. Agric. Chem. Environ., 2014, vol. 3, p. 22.
Stanley, D., Bandara, A., Fraser, S., Chambers, P.J., and Stanley, G.A., The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae, J. Appl. Microbiol., 2010, vol. 109, p. 13.
Zhang, Q., Wu, D., Lin, Y., Wang, X., Kong, H., and Tanaka, S., Substrate and product inhibition on yeast performance in ethanol fermentation, Energy Fuels, 2015, vol. 29, p. 1019.
Lin, J.-Q., Lee, S., and Koo, Y., Model development for lactic acid fermentation and parameter optimization using genetic algorithm, J. Microbiol. Biotechnol., 2004, vol. 14, p. 1163.
Peters, M.S., Timmerhaus, K.D., and West, R.E., Cost estimation, in Plant Design and Economics for Chemical Engineers, New York: McGraw-Hill, 1991, ch. 6, p. 923.
Young, D. and Cabezas, H., Designing sustainable processes with simulation: The waste reduction (WAR) algorithm, Comput. Chem. Eng., 1999, vol. 23, p. 1477.
Yaws, C.L., The Yaws Handbook of Thermodynamic Properties for Hydrocarbons and Chemicals, Beaumont, Texas: Lamar Univ., 2003.
Ulrich, G.D. and Vasudevan, P.T., How to estimate utility costs, Chem. Eng., 2006, vol. 113, p. 66.
ACKNOWLEDGMENTS
The authors express their acknowledgments to the Universidad Nacional de Colombia at Manizales and the National Call no. 761 of 2016 of the Departamento Administrativo de Ciencia, Tecnología e Innovación–Colciencias.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Serna-Loaiza, S., Ortiz-Sánchez, M., Pisarenko, Y.A. et al. Application of Thermodynamic-Topological Analysis in the Design of Biorefineries: Development of a Design Strategy. Theor Found Chem Eng 53, 166–184 (2019). https://doi.org/10.1134/S0040579519020155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579519020155