Abstract
The cathode reduction of Ce(III) ions to form a metal at an inert Mo electrode in the molten 3LiCl–2KCl eutectic is studied in a temperature range of 723–823 K by stationary and nonstationary electrochemical methods. In voltammograms, a single cathodic current peak is recorded at a potential of –3.19 ± 0.10 V, and an anodic current peak associated with the cathodic current peak is recorded at a potential of ‒3.10 ± 0.08 V vs. the chlorine reference electrode. Therefore, the reduction process occurs by the reaction Ce3+ + 3\({\bar {e}}\) → Ce. An analysis of cyclic voltammograms showed that the current peak potential of the Ce(III) ion reduction shifts to a negative range as the scan rate increases. At the same time, the current corresponding to the cathodic peak is directly proportional to the square root of the polarization rate in the entire potential range under study. An increase of the scan rate is shown to decrease the transfer coefficient (α), i.e., to increase in the irreversibility of the cathodic process. According to the theory of cyclic voltammetry, the cathode reduction of cerium ions is an irreversible process, which is single-stage and is controlled by the charge transfer rate. The temperature dependence of the apparent standard potential of the pair Ce(III)/Ce is measured by zero-current chronopotentiometry. The experimental values are described by linear equation \(E_{{{{{\text{Ce}}\left( {{\text{III}}} \right)} \mathord{\left/ {\vphantom {{{\text{Ce}}\left( {{\text{III}}} \right)} {{\text{Ce}}}}} \right. \kern-0em} {{\text{Ce}}}}}}^{{\text{*}}} = - \left( {{\text{3}}{\text{.455}} \pm {\text{0}}{\text{.010}}} \right)\) + (6.1 ± 0.1) × 10–4T ± 0.009 V. The changes in the apparent standard Gibbs energy, the enthalpy, and the entropy of the reaction of formation of cerium thrichloride from the elements in the molten 3LiCl–KCl eutectic and the activity coefficient of CeCl3 are calculated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Molten salts show promise as a medium intended for the application as a solvent in extractive metallurgy. In particular, molten chlorides are a good reactive electrolyte for the selective dissolution or precipitation of pure reagents, and the use of the molten chlorides provides an advanced direction in processing of raw materials [1].
In recent years, a new field of application of molten salts, namely, their possible application for the pyrochemical purification of main components of nuclear fuel with respect to fission products, was found. The cause of such an interest is related to the progress in estimating new concepts of transmutation of fission products in innovative fuel cycles. To estimate the possibilities of pyrochemical separation, a number of extraction processes for minor actinides in spent nuclear fuel and highly active liquid wastes [2‒6] were developed.
At present, there are conflicting data on the electrochemical behavior of cerium compounds in molten salts in the literature. The electrodeposition of metallic cerium in molten fluorides was studied in [7]. The mechanism of the process and some kinetic characteristics of cathode reduction of Ce3+ ions were determined. The results of investigation of the electrochemical properties of Ce3+ ions in the molten LiCl–KCl–CeCl3 and CaCl2–NaCl–CeCl3 systems at different electrodes in a temperature range of 450–550°C are reported in [8]. The standard rate constant k0 and the transfer coefficient α of the electrochemical reaction Ce3+ + 3\({\bar {e}}\) = Ce were calculated. The electrode reaction Ce3+ → Ce in the LiCl–KCl melt at 773 K at tungsten and liquid cadmium electrodes was studied by voltammetry in [9]. The reduction potential of Ce3+ ions at tungsten and liquid cadmium electrodes are ‒2.04 and –1.47 V vs. the AgCl/Ag reference electrode, respectively. The authors explained such a big difference in the potentials by the formation of intermetallic Ce–Cd compounds. Data on the Gibbs free energy change of the CeCd, CeCd2, CeCd3, CeCd4, and CeCd6 compounds are reported in [9]. The cathode reduction of cerium ions was studied by cyclic and square wave voltammetry in [10]. The cathode reduction of Ce3+ ions in CsCl-based melts was found to occur in two stages, namely, Ce3+ + \({\bar {e}}\) = Ce2+ and Ce2+ + 2\({\bar {e}}\) = Ce. Pourbaix diagrams were calculated and plotted in [11]. The stable oxidation forms for cerium are found to be Ce(III) and Ce(0), and Ce(IV) is stable only in the form of solid CeO2. The reduction mechanism of Ce3+ ions in the molten 3LiCl–2KCl eutectic was determined. The kinetic parameters of the process and coefficient of diffusion of Ce3+ ions were calculated. In [12], the NaCl–KCl–CeCl3 (5–30 mol %) system was studied from the viewpoint of acid-base properties. The solubilities of cerium (III) oxide and CeOCl were determined.
The aim of the present study is to investigate the mechanism of the electrochemical reduction of the cerium (III) ions in the molten 3LiCl–2KCl eutectic and to calculate the kinetic characteristics of the process and the thermodynamic properties of cerium compounds.
EXPERIMENTAL
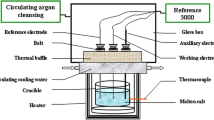
We used LiCl (Aldrich, >99.99%), reagent grade KCl (99.9%), and CeCl3 (Aldrich, 99.99%). The measurements were performed in an inert gas atmosphere using a standard three-electrode quartz cell and a glassy carbon crucible. As the working electrode, a molybdenum wire 1 mm in diameter was used, which was immersed into the melt to a depth of 3–10 mm. The surface area of cathode was determined experimentally after each experiment. As the counter electrode, a glassy carbon rod 3 mm in diameter was used; as the reference electrode, a standard chlorine electrode was used [13]. To analyze the electrochemical processes, we used the two methods, such as cyclic voltammetry and zero-current potentiometry. The measurements were performed using an AUTOLAB PGSTAT 302N potentiostat galvanostat (Eco Chemie) and NOVA 1.11 software.
RESULTS AND DISCUSSION
Figure 1 (curves 1–4) shows the cyclic voltammograms of the 3LiCl–2KCl–CeCl3 melt, which were measured at 773 K at the molybdenum inert electrode at different scan rates. In the voltammograms, a cathodic current peak is recorded at a potential of ‒3.18 V and the associated anodic current peak is recorded at a potential of –3.11 V vs. the chlorine reference electrode. An analysis of the cyclic voltammograms showed that the potential corresponding to the current peak of reduction of Ce3+ ions to Ce shifts to the negative range as the scan rate increases (Fig. 2). At the same time, the cathodic current peak is directly proportional to the square root of the polarization rate over the entire potential range under study (Fig. 3).
According to the cyclic voltammetry theory [14, 15], the cathode reduction process of cerium(III) ions to the metal occurs in single stage, which is irreversible and controlled by the charge transfer rate
The transfer coefficient (α) for electrochemical reaction (1) is calculated by the equation [14, 15]
The calculation results are given in Table 1. An increase of the scan rate is seen to cause a regular decrease of the transfer coefficient, i.e., an increase of the irreversibility of the cathodic process.
A variation in the temperature does not change the mechanism of cathode reduction of cerium(III) ions: only a regular shift in the peak potential and peak current is observed (Fig. 4).
The apparent standard potential of the Ce(III)/Ce couple at different temperatures was determined by zero-current chronopotentiometry (Fig. 5). To this end, the molybdenum cathode was polarized with the 200-mA current for 20 s and the dependence potential vs. time was then measured. The horizontal plateau of the dependence is the equilibrium potential of the Ce(III)/Ce couple. The apparent standard potential was calculated by the Nernst equation
where
The experimental values are adequately described by a linear equation, which was obtained using the least square procedure with a confidence interval of 0.95,
The apparent standard Gibbs energy change \(\left( {\Delta G_{{{\text{CeC}}{{{\text{l}}}_{{\text{3}}}}}}^{{\text{*}}}} \right)\) is determined by the equation
Since the temperature dependence of the apparent standard potential \(\left( {E_{{{\text{Ce}}\left( {{\text{III}}} \right){\text{/Ce}}}}^{*}} \right)\) is available, it is possible to determine the enthalpy \(\left( {\Delta H_{{{\text{CeC}}{{{\text{l}}}_{{\text{3}}}}}}^{*}} \right)\) and the entropy \(\left( {\Delta S_{{{\text{CeC}}{{{\text{l}}}_{{\text{3}}}}}}^{*}} \right)\) of the reaction
which is described by the equations
and
respectively.
The value of \(\Delta G_{{{\text{CeC}}{{{\text{l}}}_{3}}}}^{{\text{*}}}\) allows us to calculate the activity coefficient of \({\text{CeC}}{{{\text{l}}}_{{\text{3}}}}\left( {{{\gamma }_{{{\text{CeC}}{{{\text{l}}}_{{\text{3}}}}}}}} \right)\) in the melt by the equation
where \(\Delta G_{{{\text{CeC}}{{{\text{l}}}_{3}}}}^{o}\) corresponds to the reaction between the pure components. The activity coefficient of CeCl3 at 773 K is 4.8 × 10–3. The activity coefficients and the measured apparent standard Gibbs energy agree well with the data in [9].
CONCLUSIONS
The cathode reduction of Ce(III) ions to the metal in the molten 3LiCl–2KCl eutectic in a temperature range of 723–823 K at the inert molybdenum electrode was studied by stationary and nonstationaly electrochemical methods. The deposition mechanism of metallic cerium was determined. The cathode reduction process was found to be irreversible, to occur in a single stage by the reaction Ce3+ + 3\({\bar {e}}\) → Ce, and to be controlled by the charge transfer rate. The activity coefficients of CeCl3 in the melt were calculated, and the fundamental thermodynamic characteristics of cerium trichloride were determined.
REFERENCES
A. N. Baraboshkin, Electrocrystallization of Metals from Molten Salts (Nauka, Moscow, 1976).
A. Osipenko, A. Mayershin, V. Smolenski, A. Novoselova, and M. Kormilitsyn, Electrochemistry of curium in molten chlorides,” in Recent Trend in Electrochemical Science and Technology, Ed. by U. Kumar Sur (InTech, 2012), Chap. 1, pp. 11–30.
The Chemistry of the Actinide Elements, Ed. by J. J. Katz, G. T. Seaborg, and L. R. Morss (Chapman and Hall, London, N.-Y., 1986), Vol. 2.
Y. Fukaya, “Safety and economics of uranium utilization for nuclear power generation,” in Uranium—Safety, Resources and Thermodynamic Calculation, Ed. by N. S. Awwad (IntechOpen, 2018), Chap. 2 pp. 21–48.
J. J. Laidler, J. E. Battles, W. E. Miller, J. P. Ackerman, and E. L. Carls, “Development of pyroprocessing technology,” Prog. Nucl. Energ. 31, 131–140 (1997).
R. G. Lewin and M. T. Harrison, “International developments in electrorefining technologies for pyrochemical processing of spent nuclear fuels,” in Reprocessing and Recycling of Spent Nuclear Fuel (Woodhead Publishing, Cambridge, 2015), pp. 373–414.
V. Constantin, A.-M. Popescu, and M. Olteanu, “Electrochemical studies on cerium(III) in molten fluoride mixtures,” J. Rare Earths 28, 428–434 (2010).
Y. Castrillejo, M. R. Bermejo, D. Arocas, A. M. Martinez, and E. Barrado, “Chemical and electrochemical behavior of cerium(III) in molten LiCl–KCl and CaCl2–NaCl,” in Progress in Molten Salt Chemistry (2000), Vol. 1, pp. 143–149.
Y. Castrillejo, M. R. Bermejo, R. Pardo, and A. M. Martinez, “Use of electrochemical techniques for the study of solubilization processes of cerium-oxide compounds and recovery of the metal from molten chlorides,” J. Electroanal. Chem. 522 124–140 (2002).
S.-H. Kim, S. Paek, T.-J. Kim, D. Y. Park, and D.‑H. Ahn, “Electrode reactions of Ce3+/Ce couple in LiCl–KCl solutions containing CeCl3 at solid W and liquid Cd electrodes,” Electrochim. Acta 85, 332–335 (2012).
J. Shuqiang and Z. Hongmin, “An investigation into the electrochemical recovery of rare earth ions in a CsCl-based molten salt,” J. Hazard. Mater. 189, 821–826 (2011).
R. Combest, M. N. Levelut, and B. Tremillon, Oxoacidity and its influence on the electrochemical properties in molten mixtures of CeCl3 and equimolar NaCl–KCl at 1000 K,” Electrochim. Acta 231, 291–1295 (1978).
M. V. Smirnov, Electrode Potentials in Molten Chlorides (Nauka, Moscow, 1973.
Z. Galus, Theoretical Fundamentals of Electrochemical Analysis (Mir, Moscow, 1974).
A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications (Wiley, New York, 1980).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by N. Kolchugina
Rights and permissions
About this article
Cite this article
Novoselova, A.V., Smolenski, V.V. & Bovet, A.L. Cathode Reduction of Ce(III) Ions in Fused 3LiCl–2KCl Eutectic. Russ. Metall. 2020, 910–913 (2020). https://doi.org/10.1134/S0036029520080121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029520080121