Abstract
A study is performed of the effect pretreatment with solutions of benzoic acid in an organic solvent has on the result from the electrochemical oxidation of amorphous alloy Fe70Cr15B15 in an ionic liquid 1‑butyl-3-methylimidazolium tetrafluoroborate. It is established that the nature and properties of surface oxide films affect the result from the electrochemical oxidation of the alloy in an ionic liquid. Partial removal of oxide films by a solution of benzoic acid and subsequent electrooxidation in an ionic liquid reveals a nano-cellular structure on the electrode’s surface. It is shown that preliminarily treating the alloy with a saturated solution of benzoic acid in acetone results in a considerable anodic shift in the corrosion potential and the formation of a more effective protective coating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Amorphous multicomponent alloys based on iron attract growing attention due to their high resistance to electrochemical pitting corrosion [1]. Protective oxide films on the surfaces of alloys doped with chromium additives are not always enriched with an alloying component and often correspond in composition to the ratio of components in an alloy [2]. The presence in the alloy of the third component of a non-metallic nature (e.g., Si or B) usually enhances the corrosoin stability of steels as well [3], but can lead to destruction of the crystal structure and amorphization of the solid phase.
Amorphous alloy steels are of particular interest as materials that are compatible with biological tissues [4, 5], for which one requirement is resistance to different types of corrosion. The aim of this work was to study the electrochemical behavior of amorphous alloy Fe70Cr15B15 during its anodization in an ionic liquid (IL), 1-butyl-3-methylimidazolium tetrafluoroborate [Bmim][BF4], along with its anticorrosion properties in relation to an aquatic environment simulating the composition of physiological saline.
Due to their properties, ILs satisfy all the requirements of green chemistry. The wide window of electrochemical stability makes them particularly attractive as electrolytes. ILs based on tetrafluoroborate anions are hydrophobic and quite stable, since they do not undergo hydrolysis, which is accompanied by the formation of fluorine-containing HPO2F2, H2PO3F, H3PO4, and HF products [6].
EXPERIMENTAL
Amorphous alloy Fe70Cr15B15 was prepared from B (99.99%), Fe (99.95%), and Cr (purity, 99.95%) according to the procedure in [7]. Morphology and surface composition were studied on a Zeiss EVO-50 scanning electron microscope equipped with an energy dispersive X-ray (EDX) analyzer. X-ray phase analysis was performed on a DRON-3 X-ray diffractometer (CuKα). X-ray phase analysis revealed a fuzzy halo, testifying to the amorphous state of this alloy. The amorphous state of the alloy was confirmed via differential scanning calorimetry on a NETZSCH Jupiter STA 449 F1 unit in He. The alloy’s surface was chemicaly etched in acetone solutions of benzoic acid for 60 min. Benzoic acid with concentrations of 0.013 and 0.0065 mol/L was used for etching. Anodization was performed in IL 1-butyl-3-methylimidazolium tetrafluoroborate, using a three-electrode cell with an undivided cathode-anode space for 100 s at a current density i = 12.5 mA cm−2 using an Autolab PGSTAT302N potentiostat. The volume of the IL was 2 mL. The test sample served as the working electrode (S = 0.4 cm2), platinum and silver wires were used as auxiliary and reference electrodes, respectively. After etching, the samples were sonicated in acetone.
Corrosion studies were performed in a three-electrode cell with a divided cathode–anode space using an Autolab PGSTAT302N potentiostat. The Ringer’s solution was prepared by dissolving 6.5 g NaCl, 0.42 g KCl, and 0.25 g CaCl2 were dissolved in 1 L of distilled water [8]. The sweep rate was 1 mV/s. The solution was preliminarily purged with argon. The working and auxiliary electrodes were platinum, and the reference electrode was saturated silver–silver chloride (a CSE). The range of the studied potentials was −0.1 to 1.4 V.
RESULTS AND DISCUSSION
Three-component amorphous alloy Fe70Cr15B15 is stable in air and virtually does not corrode at ambient temperature. The surface of the material is covered with a protective oxide film whose composition is almost the same as the one between the components of the alloy (atomic ratios Fe: Cr in the alloy and the oxide (EDX) are 4.6 and 4.4, respectively). Figure 1 shows SEM images of the surface of a studied sample. The image in Fig. 1a is part of the initial sample, uniformly coated with a protective oxide film.
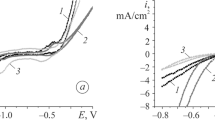
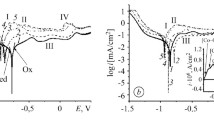
Under the anodic action in the IL, the electrode potential of the alloy first rose as a result of the electrochemical oxidation of the surface (Fig. 2). It then fell, due to destruction of the initial oxide coating (cracking). The state of the surface oxide film corresponding to the moment when part of the initial oxide coating peeled from the alloy’s surface is shown in Fig. 1b. Upon subsequent anodization of the alloy in the IL, the electrode potential grew naturally, due to an increase in film thickness resulting from the electrochemical oxidation of the alloy’s components. The extreme track of dependence E–t for the initial sample of the amorphous alloy was thus due to a change in the structure of the protective layer upon anodic exposure in the IL.
Figure 1b shows an image of a part of the surface of a sample of amorphous Fe70Cr15B15 alloy after 100 s of anodizing. It should be noted that a nanosized cellular structure appeared on the alloy’s surface as a result of anodizing the initial sample in the IL. A similar phenomenon was observed earlier on nickel and steel [9] and on Zr67Ni30Si3 alloy [10].
To clarify the role of the initial oxide film in the formation of an ordered cellular nanostructure, the surface of amorphous Fe70Cr15B15 alloy was treated with solutions of benzoic acid in an organic solvent (acetone) before anodizing in an IL. Figure 1b shows an image of part of the surface of an amorphous alloy after etching with a 0.0065 M solution of benzoic acid and subsequent anodizing in IL. As can be seen, a cellular structure emerged on the surface of the alloy on a sample pretreated with diluted benzoic acid. The self-organization of the surface of the sample therefore occurs only in the presence of the initial oxide film.
The corrosion properties of amorphous three-component alloy Fe70Cr15B15 were studied in Ringer’s solution, the composition of which mimicks physiological saline. The corrosion properties of samples pretreated with solutions of benzoic acid were also considered for purposes of comparison. The results are shown in Fig. 3. The data shows that the sample pre-treated with a concentrated (0.013 M) solution of benzoic acid, which completely destroyed the protective oxide layer, had the greatest corrosion resistance. The corrosion potential of this sample in Ringer’s solution shifts ∼0.5 V toward the anode region, relative to the original sample. On the other hand, partial removal of the oxide film of the initial sample using diluted benzoic acid had almost no effect on the corrosion potential.
CONCLUSIONS
We may assume that the electrochemical oxidation of amorphous Fe70Cr15B15 alloy in Ringer’s solution after removing the natural oxide film with benzoic acid promotes the formation of a more effective protective coating, due probably to surface benzoates of iron and chromium [11].
REFERENCES
W. B. Nowak and B. A. Okorie, Corrosion 38, 314 (1982). https://doi.org/10.5006/1.3621691
V. Paredes, E. Salvagni, E. Rodriguez, et al., J. Mater. Sci.: Mater. Med. 25, 311 (2014). https://doi.org/10.1007/s10856-013-5083-2
C. Wang, H. Zheng, H. Ding, et al., J. Alloys Compd. 766, 649 (2018). https://doi.org/10.1016/j.jallcom.2018.06.368
M. Huber, G. Reinisch, G. Trettenhahn, et al., Acta Biomater. 5, 172 (2009). https://doi.org/10.1016/j.actbio.2008.07.032
Stainless Steels for Medical and Surgical Applications, Proceedings of the Symposium, Ed. by G. L. Winters and M. J. Nutt (ASTM Int., 2003).
S. Sowmiah, V. Srinivasadesikan, M. C. Tseng, et al., Molecules 14, 3780 (2009). https://doi.org/10.3390/molecules14093780
Y. Zhijie, L. Jinfu, H. Shunrong, et al., Mater. Trans. 44, 709 (2003). https://doi.org/10.2320/matertrans.44.709
S. B. Gabriel, J. V. P. Panaino, I. D. Santos, et al., J. Alloys Compd. 536S, S208 (2012). https://doi.org/10.1016/j.jallcom.2011.11.035
O. Lebedeva, I. Kudryavtsev, D. Kultin, et al., J. Phys. Chem. C 118, 21293 (2014). https://doi.org/10.1021/jp507319r
K. B. Kalmykov, N. E. Dmitrieva, O. K. Lebedeva, N. V. Root, D. Yu. Kultin, and L. M. Kustov, Russ. Chem. Bull. 65, 2801 (2016). https://doi.org/10.1007/s11172-016-1659-6
H. M. El-Kashlan, Asian J. Chem. 23, 5235 (2011).
ACKNOWLEDGMENTS
The authors thank V.S. Snytko, I.I. Kuznetsova, and I.V. Balykova for their help in preparing this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lebedeva, O.K., Kultin, D.Y., Zakharov, A.N. et al. Electrochemical Behavior of an Amorphous Alloy in an Ionic Liquid and in Aqueous Media. Russ. J. Phys. Chem. 94, 2379–2381 (2020). https://doi.org/10.1134/S0036024420110205
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420110205