Abstract
Theoretical calculations are compared to data from an experimental study of the position of eutectics in two-component systems consisting of benzene, diphenyl, and n-alkanes with 12 to 17 carbon atoms. Data on diphenyl–n-alkane (n = 12–17) and benzene–n-alkane (n = 12–17) systems are given for the composition of eutectics, their melting points, and activity coefficients of each component. The theoretical study is based on the Schröder–Le Chatelier approach and the original UNIFAC system; the experimental study is performed via DTA. Deviations of the experimental data from ones calculated for the studied systems are presented. The change in the activity coefficients of benzene and n-hexadecane is illustrated graphically according to UNIFAC and the experimental data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
High-boiling heat-transfer fluids are used in the synthesis of various materials for industry. Heat-transfer fluids consisting of organic compounds are used at temperatures above 200°C (e.g., diphenyl, diphenyl oxide, naphthalene, and glycerol, which can maintain temperatures of 100 to 400°C) [1]. However, the melting points of such substances are high, as a result of which their crystallization in the heating circuit of the reaction apparatus is possible. If the process is conducted at low ambient temperatures, mixtures of two or more organic compounds are used as a heat carrier. For example, the Dinyl (Dowtherm A) heat-transfer fluid is widely used in the chemical industry. It is a eutectic mixture with a content of 26.5 wt % diphenyl and 73.5 wt % diphenyl oxide [1]. Dinyl is characterized by a melting point of 12.3°C, while the melting points for diphenyl ether and diphenyl are 26.84 and 68.93°C, respectively.
Works describing such systems include [2–21], but the n-alkane–aromatic hydrocarbon with two or more rings systems have been studied relatively little.
INVESTIGATION METHODS AND OBJECTS
The aim of this work was to systematize and analyze existing experimental data on benzene–n-alkane and diphenyl–n-alkane systems. The experimental data were obtained via differential thermal analysis (DTA). The experiments for each system were described in detail in [22, 23].
THEORETICAL
As in [22, 23], the characteristics of phase equilibria in the considered systems were predicted prior to experimental studies, using the Schröder–Le Chatelier equation:
where xi is the mole fraction of a component; ΔmHi is the enthalpy of melting of the component, J/mol; Tm,i is the melting point of the pure component, K; and R is the universal gas constant, 8.314 kJ/(mol K).
When calculated with the Schröder–Le Chatelier equation, the solution was considered ideal, so the activity coefficients of the components were taken as equal to 1.
This equation describes the course of the liquidus of the system from the sides of both the first and second components. The intersection of the liquidus curves gives the eutectic point. To find the eutectic, we must solve a system composed of Schröder–Le Chatelier equations with respect to xi and T:
where Te is the melting point of the eutectic composition, K.
The procedure for constructing a phase diagram using the Schröder–Le Chatelier equation was given in [24, 25].
To calculate the activity coefficients of the system’s components in the eutectic, we used a modified Schröder–Le Chatelier equation with an activity coefficient included in it:
where γi is the activity coefficient of the ith component.
The component’s activity coefficient was determined theoretically, according to UNIFAC [26]. The UNIFAC system is based on the equation
where \(\gamma _{i}^{C}\) is the combinatorial part of the activity coefficient, and \(\gamma _{i}^{R}\) is its residual part. Detailed calculations of combinatorial part \(\gamma _{i}^{C}\) and residual part \(\gamma _{i}^{R}\) were presented in [26].
The experimental values of the activity coefficients were calculated using experimental data on the liquidus temperatures of the studied mixtures. Using this approach, the activity coefficient was determined with the Schröder–Le Chatelier equation
RESULTS AND DISCUSSION
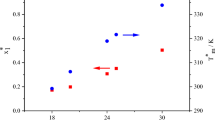
Based on our results, we compiled Tables 1 and 2, which show the values of the activity coefficients and the characteristics of the eutectics for each system.
When analyzing the activity coefficients of aromatic hydrocarbons in the studied systems, it is notable that according to the results from UNIFAC calculations, the activity coefficient grows upon an increase in the number of carbon atoms in n-alkane in systems with benzene. In contrast, the activity coefficient of diphenyl shows a drop in value according to the UNIFAC system. On the other hand, the ratios of the activities of n-alkanes fall in systems with benzene but grow in ones with diphenyl. An exception is in this case the C12H10−n-C17H36 system, where the activity coefficient falls sharply. This could be due to the proximity of the melting point of the eutectic to the that of the polymorphic transition of heptadecane. It should also be noted that in diphenyl–n-alkane systems, the activity coefficient of n-alkanes changes least when carbon atoms are added, the difference being in the third decimal place (not counting the system C12H10−n-C17H36)
The activity coefficients calculated from experimental data have multidirectional trends. For example, the activity coefficient in systems with n-C14H30 deviates from the general trend. In systems with benzene it is lower, while in systems with diphenyl it is higher than would be expected from the results for systems with n-C12H26 and n-C16H34. In systems with odd numbers of carbon atoms, only the values in system C6H6-n-C15H32 differ from the ones expected according to the results for systems with n-C13H28 and n‑C17H36.
Table 1 shows that the activity coefficients obtained using UNIFAC and those from experimental data differ greatly from one another. The percentage of the deviation of the UNIFAC data from experiments is as high as 72% (for the activity coefficient of n‑alkane in the C6H6−n-C17H36 system). However, the activity coefficient of n-heptadecane in the C12H10‒n-C17H36 system shows the maximum coincidence of the experimental and UNIFAC data: the deviation is 0.16%.
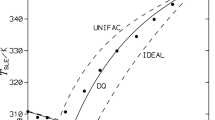
When analyzing the data in Table 2, note that in all of the considered systems, the difference between the temperature calculated with UNIFAC and the experimental one is no more than 9.24 K (system C6H6−n‑C17H36), and the deviation of the diphenyl content in the composition of the eutectic does not exceed 0.187 mole fractions (system C12H10−n-C17H36). Such a considerable difference in the content of diphenyl in the eutectic composition is probably due to the effect of polymorphism n-heptadecane, which was not considered in UNIFAC calculations. At the same time, the minimum deviation for the temperature is 0.04 K (system C12H10−n-C16H34) for a content of aromatic hydrocarbon in the eutectic of 0.0046 mole fractions (C6H6−n-C14H30). Deviations of the UNIFAC system were shown in [24].
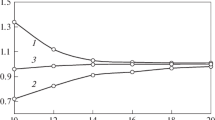
The distribution of the activity coefficients in the range of concentrations in a separate system is shown by the example of the benzene–n-hexadecane system. As can be seen from Table 3 and Figs. 1 and 2, the UNIFAC activity coefficients of the components grow along with the content of components in the mixture. At the same time, the activity coefficients calculated on the basis of experimental data show the opposite pattern and grow as the content of components falls. In addition, the experimental activity coefficients are much higher than those obtained according to UNIFAC for mixtures with low contents of one component.
CONCLUSIONS
1. UNIFAC calculations for systems consisting of diphenyl, benzene and n-alkane show small deviations in the eutectic temperature (range of errors, 0.04 to 9.24 K), but comparatively large deviations in the composition of the eutectic (deviations of up to 0.187 mole fractions). This must be considered when using this way of predicting phase equilibria in similar systems.
2. Using the above systems, we may conclude that the error of the original UNIFAC system does not depend on the number of benzene rings in the aromatic compound in an experiment. Instead, it grows in proportion to the number of carbon atoms in n-alkane in systems including n-alkanes with an odd numbers.
3. The values of the activity coefficients of the components in the eutectics of the diphenyl–n-alkane and benzene–n-alkane systems calculated according to UNIFAC and determined on the basis of the experiment differ (the calculated data deviate from the experimental ones by as much as 71.68%).
REFERENCES
A. V. Chechetkin, High Temperature Heat Carriers (Energiya, Moscow, 1971) [in Russian].
Lijiao Yu, Hong Dong, Chuan Wu, and Yindi Zhang, J. Chem. Thermodyn. 72, 139 (2014).
C. Lisa, M. Ungureanu, P. C. Cosmatchi, and G. Bolat, Thermochim. Acta 617, 76 (2015).
D. Melvin and D. Patterson, J. Solut. Chem. 8, 573 (1979).
P. Rice and S. Teja Amyn, J. Chem. Eng. Data 25, 346 (1980).
S. Sharma and M. Makavana, Fluid Phase Equilib. 375, 219 (2014).
Sheng Fang, Xiao-Bo Zuo, Xue-Jiao Xu, and Da-Hai Ren, J. Chem. Thermodyn. 68, 281 (2014).
Yindi Zhang, Hong Dong, Yan Yue, and Chuan Wu, J. Chem. Thermodyn. 57, 114 (2013).
U. Domanska and P. Morawski, Fluid Phase Equilib. 218, 57 (2004). https://doi.org/10.1016/j.fluid.2003.11.017
U. Domanska, P. Morawski, and R. Wierzbicki, Fluid Phase Equilib. 242, 154 (2006). https://doi.org/10.1016/j.fluid.2006.02.001
U. Domanska and P. Morawski, J. Chem. Eng. Data 50, 1073 (2005).
T. M. Aminabhavi, V. B. Patil, M. I. Aralaguppi, and H. T. S. Phayde, J. Chem. Eng. Data 41, 521 (1996).
J. B. Ott and J. R. Goates, J. Chem. Thermodyn. 15, 267 (1983).
J. R. Goates, J. B. Ott, and J. F. Moellmer, J. Chem. Thermodyn. 9, 249 (1977).
H. Iloukhani and M. Rezaei-Sameti, J. Chem. Eng. Data 50, 1928 (2005).
T. M. Letcher, J. Chem. Thermodyn. 7, 205 (1975).
T. M. Letcher and W. L. Spiteri, J. Chem. Thermodyn. 11, 435 (1979).
J. B. Ott, K. N. Marsh, and R. H. Stokes, J. Chem. Thermodyn. 12, 1139 (1980).
R. L. Snow, J. B. Ott, and J. R. Goates, J. Chem. Thermodyn. 18, 107 (1986).
T. Takigawa, M. Ohba, H. Ogawa, and S. Murakami, Fluid Phase Equilib. 204, 119 (2003).
N. Tripathi, Int. J. Thermophys. 26 (3), 693 (2005). https://doi.org/10.1007/s10765-005-5572-8
A. Yu. Kopnina, Cand. Sci. (Chem.) Dissertation (Samara, 2003), p. 106.
I. G. Yakovlev, Cand. Sci. (Chem.) Dissertation (Saratov, 2018), p. 156.
I. K. Garkushin, E. V. Dorokhina, and A. V. Kolyado, Butler. Soobshch. 16 (3), 41 (2009).
I. K. Garkushin, A. V. Kolyado, and E. V. Dorokhina, Calculation and Study of Phase Equilibria in Binary Systems of Organic Substances (UrO RAN, Yekaterinburg, 2011), p. 191 [in Russian].
A. G. Morachevskii, The Thermodynamics of Liquid–Vapor Equilibria (Khimiya, Leningrad, 1989), p. 344 [in Russian].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yakovlev, I.G., Garkushin, I.K. & Kolyado, A.V. Activity Coefficients of the Components of the Binary Systems Consisting of Benzene, Diphenyl, and n-Alkanes. Russ. J. Phys. Chem. 94, 1822–1826 (2020). https://doi.org/10.1134/S0036024420090320
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420090320