Abstract
Thermodynamic characteristics and water evaporation mechanism from silica structure in temperature range 100–1000°С have been studied by thermogravimetry, mass spectrometry, 1Н and 29Si NMR. It has been shown that liquid, surface-bound, and molecular-dispersed water is present on the surface and in the bulk of silica particles. The role of different (ОН)n groups in the structural change of silica upon thermal and thermal steam treatment has been shown. A method for preparation of anhydrous silica used in the manufacture of high-quality quartz glass has been proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Water in dispersed oxides is a thermodynamically complex system that depends on the properties of initial oxides (particle size, specific surface area, morphology, admixture composition, etc.) [1–5]. In turn, the properties of prepared oxide materials upon synthesis in aqueous medium, in particular on hydrothermal and thermal steam treatment, are dependent on the form of water involved in their structure. The ratio of different types of hydrogen bonds between water molecules and bonds between water molecules and elements of metal oxide scaffold plays a key role in the formation of resultant material [3–7]. For silicon dioxide, this interaction includes the binding between silanol  , silanediol
, silanediol  , silanetriol ‒Si(OH)3, and siloxane
, silanetriol ‒Si(OH)3, and siloxane  groups and water molecules on the surface and in the bulk [8, 9]. The interaction between silicon–oxygen scaffold of silica and water clusters of reaction medium are especially efficacious to control the properties of prepared materials, which fundamentally affects the most important thermodynamic parameter such as evaporation enthalpy [10]. This is due to the fact that evaporation process generally includes two processes: water molecule transfer through water–steam boundary and the formation of water molecules in liquid phase by degradation of water cluster structure [11–13]. These processes are considerably dependent on system parameters: temperature, pressure, and admixture composition; it is the cluster structure of water that extremely sensitive to admixture composition [14]. Therefore, the authors [15] revealed the dependence of water evaporation enthalpy on admixtures at the level of 10–4 wt % when studied the properties of “anomalous” water. Admixture composition has a dramatic effect on “anomalous” water properties, including viscosity and thermal conductivity [15], which at present provides a foundation to develop new scientific and engineering approach to the synthesis and study the properties of nanofluids. Nanofluids are microsuspensions containing admixtures in the range from 10–4 to 1 wt % with average particle size about 40 nm. The properties of nanofluids may reach 50% of tabulated values [16–20]. In this context, there is generality between water properties in disperse oxides and water properties in nanofluids, which attracts attention to these subjects and causes numerous publications in this field [1–9, 16–20]. Thus, the work [16] reported the dependence of thermal conductivity and viscosity of nanofluids on nanoparticle concentration. It was found that the thermal conductivity and viscosity of nanofluids increase with nanoparticle concentration.
groups and water molecules on the surface and in the bulk [8, 9]. The interaction between silicon–oxygen scaffold of silica and water clusters of reaction medium are especially efficacious to control the properties of prepared materials, which fundamentally affects the most important thermodynamic parameter such as evaporation enthalpy [10]. This is due to the fact that evaporation process generally includes two processes: water molecule transfer through water–steam boundary and the formation of water molecules in liquid phase by degradation of water cluster structure [11–13]. These processes are considerably dependent on system parameters: temperature, pressure, and admixture composition; it is the cluster structure of water that extremely sensitive to admixture composition [14]. Therefore, the authors [15] revealed the dependence of water evaporation enthalpy on admixtures at the level of 10–4 wt % when studied the properties of “anomalous” water. Admixture composition has a dramatic effect on “anomalous” water properties, including viscosity and thermal conductivity [15], which at present provides a foundation to develop new scientific and engineering approach to the synthesis and study the properties of nanofluids. Nanofluids are microsuspensions containing admixtures in the range from 10–4 to 1 wt % with average particle size about 40 nm. The properties of nanofluids may reach 50% of tabulated values [16–20]. In this context, there is generality between water properties in disperse oxides and water properties in nanofluids, which attracts attention to these subjects and causes numerous publications in this field [1–9, 16–20]. Thus, the work [16] reported the dependence of thermal conductivity and viscosity of nanofluids on nanoparticle concentration. It was found that the thermal conductivity and viscosity of nanofluids increase with nanoparticle concentration.
The authors [16] studied by TGA the change in enthalpy of water evaporation from nanofluids containing Al2O3 and TiO2 nanoparticles in the range 0.01–1 wt % with particle size of 13, 20, and 80 nm. It was found that the higher volume concentration of nanofluid and larger Al2O3 nanoparticles, the lower value of water evaporation enthalpy. Similar dependence is observed for nanofluids containing TiO2 particles.
Considerable interest is also attracted to the study of physicochemical properties of water in thin layers adjacent to phase boundary, which is called as surface-bound water. Its properties are considerably different from those of common liquid water. Interest in this kind of water is determined by its role in physicochemical processes (adsorption, catalysis, colloidal processes), but its role in biological processes is the most important. The properties of surface-bound water were theoretically and experimentally studied in the works [1–7, 10, 21]. The role of surface-bound water in biological systems was considered in the works [21–23]. The difference of properties of surface-bound water from that of bulk water is determined mainly by the properties of surface, the presence of polar groups capable of hydrogen bonding, the presence of surface ions, surface geometry, etc. Rearrangement of 3D network of water structure proceeds under the action of surface forces. Modification of water layer is determined by the nature and number of surface atoms capable of hydrogen bonding with water molecules.
The properties of surface-bound water (heat capacity, viscosity, density, electrical conductivity, selective adsorption) were studied in the works [3–7, 10]. The most interesting is the direct experimental measurement of thermal properties of surface-bound water, in particular, activation enthalpy and energy of evaporation, however, there are few such experiments owing to considerable experimental difficulties. Vapor pressure over surface of water column was determined in the work [15] for structurally modified water obtained in quartz capillary tubes and a considerable decrease of equilibrium vapor pressure was detected. Evaporation heat determined from the temperature dependence of liquid column length at 200°С was 6 ± 1 kcal/mol on average. Based on the analysis of experimental data for the physical properties of surface-bound water, a model of its structure was proposed in the works [24–26]. The model is based on the bimodal distribution function of enthalpy for liquid water calculated from experimental data for heat capacity. The enthalpy distribution is a bimodal function with maxima at ~1 and 3 kcal/mol, which allowed the author to postulate two-structural model of liquid water and consider that surface-bound water is enriched with ice-like component where fraction of molecules bound by four hydrogen bonds rises from 0.1 in bulk water to 0.4 in bound water [25].
Silica (silicon dioxide SiO2) is the inorganic polymer of corpuscular structure. For silica crystalline modifications, valence angles between silicon and oxygen atoms are constant and determine the structure and properties of these modifications. At the same time, the values of valence angles for amorphous silica vary within 120°–180°, which in combination with the presence of localized water molecules leads to disordering of its structure. The properties of amorphous silica are determined mainly by the character of supramolecular species: the size of flocculi, their symmetry, structure regularity, etc. The presence of water in silica structure is related to both preparation method and change in its structure on subsequent treatment. The interaction of water with hydroxyl groups on the surface and in silica bulk leads to its structural modification, i.e., to the formation of surface-bound water. Thus, amorphous silica may contain the following structural elements: silicon–oxygen scaffold produced by 3D network of siloxane bonds, hydroxyl groups (ОН)n at silicon atoms, and water molecules located in different regions of silicon-oxygen scaffold.
To date, there is no unambiguous depiction of hydrated silica structure. In spite of the large number of studies dedicated to this issue [27–31], the structure, location, and character of localization of water on the surface and in the bulk of silica, relative number of silanol, silanediol, silanetriol, and siloxane groups, the coordination of silicon atoms containing (ОН)n groups, the character of mutual perturbation of silanol groups and their interaction with water molecules or clusters remain unclear.
This work deals with the study of water species on the surface and in the bulk of hydrated silicon dioxide and determination of the role of different (ОН)n groups during structural change of silica on thermal and thermal steam treatment.
EXPERIMENTAL
Chemicals used in the study were silicic acid (analytical grade), silica (Angarsk chemical plant) (high purity grade 14-4), silica samples prepared by hydrolysis of chemicals manufactured by Khimprom Production Corporation (Novocheboksarsk): sodium silicate (Na2SiO3), silicon tetrachloride (SiCl4), and tetraethoxysilane (C2H5O)4Si. To normalize the content of hydroxyl groups and water, samples were heated in air in the range 100–1500°С and exposed to hydrothermal and thermal steam treatment in laboratory autoclaves at 150–350°С.
Thermogravimetric study was performed on a 1000D MOM and a Rigaku Corporation derivatographs at heating rate of 10 K/min in the temperature range 20–1000°С. Reference used was Al2O3. IR spectra were recorded on an UR-20 and a Specord spectrometers in the range 400–4000 cm–1 as KBr pellets. 1H NMR spectra were obtained on a Bruker WP-80 pulsed spectrometer operating at 80 MHz at ambient temperature (300 K) using trichloromethane-d as a solvent and internal reference; 29Si NMR spectra with 29Si–1Н cross polarization with proton decoupling during signal recording and sample spinning at the magic angle were recorded on a Bruker MSL-300 spectrometer operating at 15.69 MHz using trichloromethane-d as a solvent and internal reference. All chemical shifts are given relative to the signal of tetramethylsilane as external reference without correction for volume magnetic susceptibility. Measurement procedure was reported in the works [10, 32].
RESULTS AND DISCUSSION
To identify different forms of hydrated silica and different water forms in its structure, the main issue is the coordination number of silicon atom in silicon–oxygen scaffold. It is debatable question. In the authors opinion [33, 34], in the case of localization of water molecules at silicon atoms producing both siloxane and silanol groups, silicon coordination number should be larger as compared with aprotic oxygen–silicon compounds. The authors believe that one can expect silicon CN = 5 or 6 in silicon aqua hydroxo complexes.
Our 29Si NMR study showed that no silicon coordination different from four was detected within method sensitivity irrespective of the method of preparation and subsequent silica treatment. For the samples of aqueous amorphous silica prepared by hydrolysis (SiCl4, Na2SiO3, and (C2H5O)4Si), 29Si NMR spectra display three signals with chemical shifts of –91, –100, and –109 ppm (Fig. 1, curve 1), which refer to silanediol, silanetriol, and siloxane groups, respectively.
Neither silanetriol groups nor orthosilicic acid Si(ОН)4 molecules were detected in any sample. Obviously, only silicon atoms with CN = 4 are present both on surface and in the bulk of silica. The spectra of samples heated in air at 1000°С show only two signals with chemical shifts –100 and –109 ppm (Fig. 2, curve 2), which indicates the disappearance of silanediol groups and increase in the relative number of siloxane bonds. Thermal steam treatment has the same effect (Fig. 1, curve 3). One can suppose that the disappearance of silanol groups accompanied by increase in siloxane bonds proceeds due to reaction of silanediol groups with neighboring silanol or silanediol groups (Scheme 1). Next, neighboring silanediol groups recombine to form siloxane bonds.

Scheme 1 .
Silica samples for thermogravimetric studies were obtained by hydrolysis of different precursors (SiCl4, Na2SiO3, and (C2H5O)4Si). The samples were washed with distilled water and dried in air at 60°С. In all cases, thermogravimetric curves showed considerable extended endothermal effect in the range 90–180°С with maximum at 135–140°С, which is accompanied by the weight loss of 5–8 wt % (Table 1). Evaporation enthalpy was 8.5–9.5 kcal/mol of evaporated water, which is slightly lower than evaporation enthalpy for liquid water (10 kcal/mol). One can suppose that this range includes the evaporation of different water forms, which causes lower value of evaporation enthalpy as compared with liquid water.
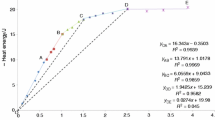
To verify this assumption, we performed layer-by-layer water evaporation with simultaneous determination of evaporation enthalpy. Figure 2 displays thermogravimetric curve for water evaporation from silicon dioxide obtained by hydrolysis of sodium silicate with specific surface area of 180 m2/g.
Figure 2 shows that evaporation enthalpy is 10 kcal/mol for the sample of silicon dioxide containing 9 wt % of water upon water layer evaporation from open pores up to 1 wt % water, which indicates the evaporation of liquid water. Starting from the layer of 1 wt % water, evaporation enthalpy is 5 kcal/mol, which enables one to draw a conclusion that this range includes evaporation of water bound by hydrogen bonds with silanol and silanediol groups on the surface of silicon dioxide.
To study water state in the bulk of silicon dioxide, the studied samples were subjected to hydrothermal treatment in autoclave at 180°C in 5 wt % ammonium hydroxide solution for 6 h. For this sample, DTA curves showed three endothermal effects with maxima at 100, 400, and 500°С. Evaporation enthalpy for different water forms was 8, 5, and 1 kcal/mol. Obviously, the dehydration of modified samples of silica proceeds in three temperature ranges: 50–120, 250–500, and 500–1000°С and is accompanied by endothermal effects and marked weight loss.
Water evaporation from open silica gel pores proceeds in temperature range 50–120°С. The total amount of water evaporated in this temperature range is ~6 wt % of sample weight. The integral heat of evaporation is ~10 kcal/mol, while activation energy of evaporation determined from gravimetric curves by non-isothermal kinetics under linear temperature growth is ~10 kcal/mol. These data indicate that thermodynamically inhomogeneous liquid water undergoes evaporation in this temperature range. Thus, after evaporation of the main water amount, the heat of evaporation for remaining layer, which constitutes 1% of sample weight, is ~6 kcal/mol and corresponds to the heat of evaporation of liquid water (Fig. 2). It is convenient to study evaporation in the second temperature range on the samples subjected to hydrothermal or thermal steam treatment. Three thermal effects of 10, 5, and 1 kcal/mol were detected for these samples were obtained at 250, 350, and 500°С, respectively. Evaporation of surface water completes after heating for 3 h at 150°С.
For initial samples containing ~6 wt % water, 1Н NMR spectrum under magic angle shows only one line with chemical shift of 5 ppm, while IR spectrum displays one wide band in the range 3200–3800 cm–1. After heating for 3 h at 150°С, 1Н NMR spectrum exhibits a wide line with chemical shift of 2 ppm; IR spectrum displays three wide resolved bands with maxima at 3200, 3450, and 3680 cm–1. 29Si NMR spectrum is identical to that of the initial sample. Taking into account that the chemical shift of liquid water on silica surface is 5 ppm, the line with chemical shift of 2 ppm may refer to surface-bound water in globule bulk. One can suppose that structural water modification in silica bulk is determined by silanediol groups, whose presence is evidenced by the line with chemical shift of –90 ppm in 29Si NMR spectrum.
For samples subjected to hydrothermal treatment, 1Н NMR spectrum shows two lines with chemical shifts of 5 and 3 ppm. The heating of these samples for 3 h at 400°С leads to disappearance of the line at 5 ppm. Only one line at 3 ppm remains in the spectrum, which may be referred to surface-bound water formed due to modification of liquid water with silanol and siloxane groups. It is significant that these samples (like in silica heated at 1000°С) show in 29Si NMR spectrum only two signals with chemical shifts of –100 and –109 ppm of almost the same intensity due to silanol and siloxane groups.
The 1Н NMR spectra of samples heated at 1300°С show only one line with chemical shift ~1 ppm, while IR spectrum displays one band at 3680 cm–1. Since the absorption of isolated hydroxyl groups in IR spectrum is characterized by the band at 3750 см–1 [10], one can draw a conclusion that high-temperature thermal treatment leads to formation of silanol groups, which are associated with neighboring water molecules bound by two hydrogen bonds with the proton of the silanol group and oxygen atom of siloxane bond, rather than free silanol groups.
Our study showed that water structurally modified by surface forces is located both on the surface and in the bulk of particles of amorphous hydrates silica along with hydroxyl groups and liquid water. The structural modification of water is determined by the presence of polar hydroxyl groups both on the surface and in the bulk of silica particles. Depending on the number of hydroxyl groups at silicon atom (silanol, silanediol), the character of structural modification may vary significantly. The interaction of water molecules with hydroxyl groups at silicon atoms leads to formation of clusters of surface-bound water with slightly lower heat of evaporation as compared with the heat of evaporation of liquid water (~6 kcal/mol). This form of water can be located both on the surface of silica particles (when water content is not higher than 1 wt %) and in the bulk of its particles. When silica structure is partially dehydroxylated due to removal of first of all silanediol groups, the character of water modification changes and is determined by interaction with silanol hydroxyl groups. For this form of water, the heat of evaporation is considerably lower and varies in the range 1–3 kcal/mol. Thus, high activation energy is typical for bound water in the bulk of silica particles, which indicates the relative difficulty of water diffusion from the bulk of silica particles.
It was shown in the works [35–39] by computer experiment method that the local water density in the volume confined by flat surfaces is oscillated along direction perpendicular to the confined surface, attenuating at a certain distance from solid support, which can result in change in hydrogen bonding structure and, therefore, to water modification. Continual water structure transfers to discrete cluster state where cluster sizes are determined by homogeneity domains. Hydrogen bonds of water molecules in one layer have larger energy than those in different layers.
The results of study of thermodynamic properties of water clusters were reported in the works [35, 36]. Formation enthalpy of water dimer determined by mass-spectral method is 4.2 kcal/mol, while the average enthalpy per one water molecule is 2.1 kcal/mol. The enthalpy of addition of water molecule to dimer is 6.7 kcal/mol, while the average enthalpy of one molecule in trimer is 3.6 kcal/mol, which is by factor 1.7 higher than that for the dimer. The average enthalpy of one molecule is 8.4 kcal/mol for tetramer and 9.4 kcal/mol for pentamer, which is close to evaporation enthalpy for pure water.
The comparison of our results with available literature data shows that hydrated silica contains liquid water on the surface (in open pores). Upon heating a sample, as water escapes down to content of ~1 wt %, it undergoes modification owing to surface hydroxyl groups. The value of evaporation enthalpy of 6 kcal/mol obtained by us allows us to conclude on the basis of the works [35–37] that water is present on silica surface as dimers at content lower than 1 wt %. The bulk of silica particles probably contains clusters with different number of water molecules, which is evidenced by the presence of three resolved bands in IR spectrum (3200, 3450, and 3680 cm–1) in the region of stretching vibrations of hydroxyl groups and a strong band at 1640 cm–1 related to the deformational vibrations of water molecules. Proton exchange between water molecules and hydroxyl groups determines the averaged chemical shift of 2 ppm in 1H NMR spectrum. The removal of isolated water molecules bound to silanol and siloxane groups from the bulk is kinetically very difficult, which is evidenced by the high value of evaporation activation energy. Therefore, this form of water is present in silica up to its melting and even in quartz glass (a band at 3680 cm–1). At the same time, the low value of evaporation enthalpy (~1 kcal/mol at 500°С) indicates the relatively weak binding of these molecules in the bulk of silica particles. Thus, the bulk of silica particles can include liquid water, surface-bound water as clusters, and water in molecule-dispersed state, i.e., bound by hydrogen bonds with protons of silanol groups and oxygen of siloxane bonds.
The structure of silica surface hydrate cover is of great importance for such processes as catalysis and adsorption, whereas the energetic state of water in the bulk of particles play a key role in the processes of silica structure modifications upon thermal and thermal steam treatment. The obtained results are important for the preparation of new silica-based materials with prescribed properties.
CONCLUSIONS
Enthalpy and mechanism of water evaporation from silica structure in the range 100–1300°С was studied. It was shown that liquid, surface-bound, and molecule-dispersed water is present on the surface and in the bulk of silica particles. The role of different (ОН)n groups in the process of silica structure change upon thermal and thermal steam treatment was shown.
REFERENCES
G. P. Panasyuk, I. V. Kozerozhets, E. A. Semenov, et al., Inorg. Mater. 55, 929 (2019). https://doi.org/10.1134/S0020168519090139
G. P. Panasyuk, I. V. Kozerozhets, E. A. Semenov, et al., Inorg. Mater. 55, 920 (2019). https://doi.org/10.1134/S0020168519090127
I. V. Kozerozhets, G. P. Panasyuk, E. A. Semenov, et al., Russ. J. Inorg. Chem. 65, 1529 (2020). https://doi.org/10.1134/S0036023620100149
I. V. Kozerozhets, G. P. Panasyuk, E. A. Semenov, et al., Russ. J. Inorg. Chem. 65, 1384 (2020). https://doi.org/10.1134/S0036023620090090
I. V. Kozerozhets, G. P. Panasyuk, E. A. Semenov, et al., Inorg. Mater. 56, 716 (2020). https://doi.org/10.1134/S0020168520070092
I. V. Kozerozhets, G. P. Panasyuk, E. A. Semenov, et al., Theor. Found. Chem. Eng. 54, 465 (2013). https://doi.org/10.1134/S0040579520030082
G. P. Panasyuk, V. N. Belan, I. L. Voroshilov, et al., Theor. Found. Chem. Eng. 47, 415 (2013). https://doi.org/10.1134/S0040579513040143
M. W. Mutahi, T. Nittoli, L. X. Guo, et al., J. Am. Chem. Soc. 124, 7363 (2002). https://doi.org/10.1021/ja026158w
V. V. Libanov, A. A. Kapustina, N. P. Shapkin, et al., Silicon 11, 1489 (2019). https://doi.org/10.1007/s12633-018-9969-y
M. N. Danchevskaya, V. A. Kreisberg, V. R. Rakcheev, et al., Inorg. Mater. 35, 1060 (1999).
S. Okada, S. Ohsaki, H. Nakamura, et al., Chem. Eng. Sci. 227, 115938 (2020). https://doi.org/10.1016/j.ces.2020.115938
F. Berthias, L. Feketeova, H. Abdoul-Carime, et al., Phys. Chem. Chem. Phys. 20, 18066 (2018). https://doi.org/10.1039/c8cp02657b
F. Calvo, F. Berthias, L. Feketeova, et al., Eur. Phys. J. D 71, 110 (2017). https://doi.org/10.1140/epjd/e2017-80062-5
K. Chatterjee and O. Dopfer, Phys. Chem. Chem. Phys. 21, 25226 (2018). https://doi.org/10.1039/c9cp05042f
B. Derjaguin and N. Churaev, Nature 244, 430 (1973). https://doi.org/10.1038/244430a0
C. Y. Tso and Y. H. Chao Christopher, Int. J. Heat Mass Transfer 84, 931 (2015). https://doi.org/10.1016/j.ijheatmasstransfer.2015.01.090
C. Y. Tso, S. C. Fu, and Y. H. Chao Christopher, Int. J. Heat Mass Transfer 70, 202 (2014). https://doi.org/10.1016/j.ijheatmasstransfer.2013.10.077
C. Y. Tso, K. C. Chan, Y. H. Chao Christopher, et al., Int. J. Heat Mass Transfer 85, 343 (2015). https://doi.org/10.1016/10.1016/j.ijheatmasstransfer.2015.02.005
H. H. Lee, S. C. Fu, C. Y. Tso, et al., Int. J. Heat Mass Transfer 105, 230 (2017). https://doi.org/10.1016/j.ijheatmasstransfer.2016.09.093
F. R. Siddiqui, C. Y. Tso, S. C. Fu, et al., Int. J. Heat Mass Transfer 153, 119618 (2020). https://doi.org/10.1016/j.ijheatmasstransfer.2020.119618
Q. L. Ye, R. Eves, R. L. Campbell, et al., Biochem. J. 477, 3271 (2020). https://doi.org/10.1042/BCJ20200539
T. Igarashi, M. Hoshi, K. Nakamura, et al., J. Phys. Chem. 124, 4196 (2020). https://doi.org/10.1021/acs.jpcc.0c00423
A. R. Symington, M. Molinari, S. Moxon, et al., J. Phys. Chem. 124, 3577 (2020). https://doi.org/10.1021/acs.jpcc.9b09046
H. J. Bakker and J. L. Skinner, Chem. Rev. 110, 1498 (2010). https://doi.org/10.1021/cr9001879
G. M. Mantrova, Biomed. Radioelektron. 7, 58 (1999).
I. Napari and A. Laaksonen, J. Chem. Phys. 111, 5485 (1999). https://doi.org/10.1063/1.479809
Y. Zhou, H. J. Zheng, Y. W. Qiu, et al., Front. Mater. 7, 127 (2020). https://doi.org/10.3389/fmats.2020.00127
A. Cretu, C. Mattea, S. Stapf, et al., Mol. Phys. 117, 1006 (2019). https://doi.org/10.1080/00268976.2018.1513581
Y. P. Wu and T. Wang, J. Colloid Interface Sci. 448, 100 (2015). https://doi.org/10.1016/j.jcis.2015.02.020
B. Rager and A. Krysztafkiewicz, Colloids Surf., A 125, 121 (1997). https://doi.org/10.1016/S0927-7757(97)00063-0
V. V. Turov, R. Leboda, and V. I. Bogillo, Langmuir 13, 1237 (1997). https://doi.org/10.1021/la951565p
V. B. Lazarev, G. P. Panasyuk, I. L. Voroshilov, et al., Ind. Eng. Chem. Res. 35, 3721 (1996). https://doi.org/10.1021/ie950404d
N. He, H. B. Xie, and Y. H. Ding, J. Comput. Chem. 29, 1850 (2008). https://doi.org/10.1002/jcc.20959
Y. Z. Chen, X. L. Feng, J. Chen, et al., Phys. Rev. B 99, 184106 (2019). https://doi.org/10.1103/PhysRevB.99.184106
S. K. Reddy, S. C. Straight, P. Bajaj, et al., J. Chem. Phys. 145, 194504 (2016). https://doi.org/10.1063/1.4967719
S. V. Shevkunov, Colloid J. 78, 257 (2016). https://doi.org/10.1134/S1061933X16020137
S. Karthikeyan and K. S. Kim, Mol. Phys. 107, 1169 (2009). https://doi.org/10.1080/00268970902784900
X. H. Ju, J. J. Xiao, and H. M. Xiao, J. Mol. Struct.: THEOCHEM 626, 231 (2003). https://doi.org/10.1016/S0166-1280(03)00124-6
V. A. Kreisberg, Y. D. Ivakin, and M. N. Danchevskaya, J. Eur. Ceram. Soc. 39, 508 (2019). https://doi.org/10.1016/j.jeurceramsoc.2018.09.031
ACKNOWLEDGMENTS
This work was performed under the State Assignment in the field of basic research for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated by I. Kudryavtsev
Rights and permissions
About this article
Cite this article
Panasyuk, G.P., Kozerozhets, I.V., Voroshilov, I.L. et al. Water Forms on the Surface and in the Bulk of Silicon Dioxide. Russ. J. Inorg. Chem. 66, 724–730 (2021). https://doi.org/10.1134/S0036023621050120
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621050120