Abstract
A cadmium(II) coordination polymer (CP), namely [Cd(H2L2–)(2,2′-bipy)]n (1) based on 3‑(3′,5′-dicarboxyphenyl)pyridine-2,6-dicarboxylic acid, has been synthesized under hydrothermal conditions. Compound 1 crystallizes in the orthorhombic system Pbca space group and features a 1D infinite Zigzag chain structure, further extending into a 3D supramolecular network through O–H\( \cdots \)O hydrogen bond interactions. CP 1 exhibits highly selective and sensitive luminescent detection for nitrophenol in ethanol solution with quenching efficiency up to 2.84 × 104 M–1 and a low detection limit of 4.4 μM. Remarkably, CP 1 might be a potential luminescent sensor material for nitrophenol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Coordination polymers (CPs), as a class of multifunctional materials assembled from using coordination bonds between organic multi-carboxylic acid ligands and metal ions or clusters, have attracted great interest because of their promising applications in magnetism, catalysis, ion exchange, nonlinear optics and other fields [1–8]. Especially, luminescent coordination polymers (LCPs) have been considered as potential luminescent materials, which have already been applied in the selective and sensitive detection of environmental pollutants including organic dyes [9–15], heavy metal ions [16–20], nitroaromatics (NACs) [21–24], and other contaminants [25–29].
With rapid development of industrialization, NACs pollutants have attracted significant attention. Rapid detection of nitroaromatics has the vital significance for homeland security and environmental safety [30]. Nitrophenol (NP) as raw materials has been widely utilized in many fields. The rivers, groundwater and soils are heavily contaminated by the excessive release of NP [31–33]. Therefore, it is indispensable to quickly and effectively detect NP. However, the traditional detection methods are expensive, complicated and time-consuming, together with other sophisticated instruments at the time [34–36]. LCPs have been considered as excellent chemosensors to sense nitroaromatics on account of the unique features of simple and environmental friendly generation of fluorescence quantum, with the addition of the lower detection limit as well as the selective identification of targeted molecules.
In this work, 3-(3′,5′-dicarboxyphenyl)pyridine-2,6-dicarboxylic acid (H4L) was selected to form novel CP. To the best of our knowledge, Cd(II) was often employed to construct LCPs due to its intrinsic electronic configuration, leading to form various types of sophisticated coordination architectures, including grids, helicates, and clusters [37–40]. Herein, a new luminescent CPs, namely [Cd(H2L2–)(2,2′-bipy)]n (1) was obtained under hydrothermal conditions. CP 1 exhibits a 1D infinite Zigzag chain structure and can be connected by hydrogen bond to form a 3D supramolecular structure. The luminescent intensity of 1 can be quenched by NP. Fluorescence titration experiments confirmed that it has excellent potential in sensing NP. In addition, the possible quenching mechanism was discussed deeply.
EXPERIMENTAL
Chemicals and Instrumentation
All of the chemicals and solvents are commercially available and were utilized without any further purification. The H4L ligand was purchased from Jinan Camolai Trading Company. The infrared spectra were measured on a Bruker Tensor 27 IR spectrometer using KBr pellets in the range 4000–400 cm–1. Powder X-ray diffraction (PXRD) patterns were recorded on a Rigaku MiniFlex 600 diffractometer with CuKα radiation (λ = 1.5406 Å). Thermogravimetric analyses (TGA) were recorded on a Netzsch TG 209 TG–DTA analyzer at a heating rate of 10°C/min from room temperature to 600°C under flowing nitrogen atmosphere. Elemental analyses (C, H, and N) were carried out on a Perkin-Elmer 240 elemental analyzer. UV–vis spectra were measured on a JASCO V-570 spectrophotometer. The fluorescent spectra were recorded on an F‑4500 FL spectrophotometer.
Synthesis of [Cd(H2L2–)(2,2′-bipy)]n (1)
A mixture of Cd(NO3)2 · 4H2O (30.8 mg, 0.1 mmol), 2,2′-bipy (15.6 mg, 0.1 mmol) and H4L (33.1 mg, 0.1 mmol) was mixed in 10 mL of H2O. The final mixture was sealed in a 23 mL Teflon reactor and heated at 130°C for 3 days, followed by cooling to room temperature at a rate of 10 K/h. Colorless crystals were collected (19.4 mg, 47% yield). Anal. calcd. for C25H15CdN3O8 (1): C 50.23, H 2.53, N 7.03%. Found: C 50.64, H 2.92, N 7.46%. FT-IR (KBr, cm–1): 3536(m), 3406(m), 2604(m), 1708(s), 1637(s), 1561(m), 1483(m), 1402(m), 1348(s), 1243(m), 1196(m), 1114(w), 1027(w), 926(m), 852(w), 763(m), 698(m), 663(m), 527(w), 474(w).
X-ray Crystallography
The crystallographic data for compound 1 were collected on a Rigaku Saturn CCD diffractometer equipped with graphite-monochromated MoKα radiation (λ = 0.71073 Å). The structures were solved by the direct methods and refined by the fullmatrix least-squares method on F2 (SHELXTL-2014) [41]. All nonhydrogen atoms were located from the Fourier maps and were refined with anisotropic displacement parameters. Hydrogen atoms of water molecules were found from different Fourier maps and then refined with isotropic temperature factors. Hydrogen atoms attached to carbon atoms were placed on calculated positions, and their positions were refined using a riding model. The pertinent crystallographic data and structure refinement parameters for compound 1 are listed in Table 1. The selected bond lengths and angles of 1 are given in Table S1. The CCDC number of 1 is 1847039.
Fluorescence Titrations
The luminescence mechanisms of 1 have been researched in the solid state and in various solvent molecules at ambient temperature. The preparation process of solvent emulsions was introduced 2 mg of 1 crystalline powder into 3.0 mL of different solvents, including methanol (MeOH), ethanol (EtOH), H2O, CH3CN, CH2Cl2, benzene, toluene, nitrobenzene (NB) and NP, and ultrasonicated for 30 min, forming stable suspensions for fluorescence study. Different amounts of 0.001 M NP were introduced into EtOH emulsion for sensing properties with regard to NP. Meanwhile, the reproducibility of 1 toward sensing NP has been researched in detail, the powder of 1 was recovered by centrifugation and washed with EtOH after the first quenching experiment, which was collected and used in the successive quenching experiments.
RESULTS AND DISCUSSION
Description of the Crystal Structure
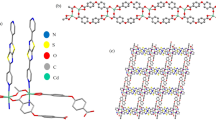
Compound 1 crystallizes in orthorhombic system, Pbca space group. The asymmetric unit of 1 includes one independent Cd(II) atom, one undeprotonated H2L2– anion ligand, and one 2,2′-bipy molecule, as illustrated in Fig. 1a. The central Cd2+ ion is in a slightly distorted trigonal prism geometry, coordinated by three N atoms from one H2L2– ligand and 2,2′-bipy molecule and three O atoms from two different H2L2– ligands with the Cd–O distances in the range of 2.2340(13)–2.4214(14) Å and Cd–N distance is between 2.2744(14) and 2.3723(15) Å. Interestingly, the undeprotonated H2L2– anion ligand bridges two Cd atoms to form a 1D infinite Zigzag chain (Figs. 1b and S1), which are further built 2D networks by hydrogen-bonding interactions (Figs. 1e). Moreover, 3D supramolecalar architecture was generated by the weakly hydrogen bonding interactions (Fig. 1f and S2).
Representation of the coordination environments of the Cd2+ centre (symmetry code: #1 x − 1/2, y, −z + 1/2) (a), 1D infinite Zigzag chain structure of 1 (b). Schematic diagram of hydrogen bonds (c, d). View of 2D networks stabilized by C–H\( \cdots \)O hydrogen bonds (e). View of 3D supramolecular frameworks (f).
TGA and PXRD
To characterize the thermal stabilities of CP 1, thermal behavior was investigated by TGA (Fig. S3). CP 1 is stable up to ca. 228°C and then the decomposition of the framework occurs. The powder X-ray diffraction (PXRD) analyses were performed on polycrystalline samples of CP 1 (Fig. S4). The experimental patterns are consistent with the simulated ones from single crystal X-ray data, illustrating that CP 1 are pure samples.
Luminescent Properties
Solid state emission spectral analysis for CP 1 and H4L ligand were performed at room temperature. (Fig. S5). The maximum emission at 437 nm for 454 nm are the characteristic emissions of ligand and CP 1, respectively. Cd(II) is difficult to oxidize or reduce due to its d10 electronic configuration. Thus, the luminescent behavior of CP 1 can be assigned to the charge transfer (π → π* and n → π*) of internal free ligands.
Sensitive Luminescent Detections
The luminescent behaviors of CP 1 in different solvents or analytes were investigated. As shown in Fig. 2, CP 1 exhibits the varying emission intensities in diverse solvents or analytes, owing to the types of solvents or analytes. The emission intensity of CP 1 in different solvents gives a sequence of solution: EtOH > MeOH > H2O > CH3CN > CH2Cl2 > toluene > benzene > NB > NP. Significantly, NP exists the largest quenching effect on the emission of CP 1, while the others show a different decrease. The PXRD patterns further demonstrated that the crystallinity of CP 1 was integrated after immersing in different solvents (Fig. S6), indicating the highly selective detection and recognition of NP. Furthermore, titration experiments were performed to explore the detection limit of CP 1 as a luminescent probe. With the increase of concentration of NP, the luminescence intensity of CP 1 can weaken accordingly. As illustrated in Fig. 3, the luminescence intensity gradually decreases by adding different amounts of EtOH solutions of NP. The luminescence intensity of CP 1 almost completely quenched when 150 μL NP solutions added. Additionally, the linear luminescence intensity vs. NP concentration plot was calculated using the Stern–Volmer equation: I0/I = Ksv[A] + 1, which is conducted to illustrate the relationship between the quenching effect and NP concentration. The Ksv value was calculated to be 2.84 × 104 M–1, indicating the high quenching efficiency of NP in the emission of CP 1. Besides, the detection limit of CP 1 was calculated using the equation: DL = 3σ/K (1), σ = 100 (S/I0) (K is the slope between the luminescence intensity vs. the NP concentration) [42–48]. The detection limit was as low as 4.4 μM (S/N = 3), which is comparable to that of the reported CPs [31–33]. This result displays that CP 1 as a visually luminescent probe becomes possible for discrimination and detection of NP. Significantly, the tested samples possess superb recyclability (at least five times) (Fig. 3d). These results reveal that CP 1 can be acted as a luminescent sensing material.
Luminescence spectra of 1 with different concentrations of NP in DMF solutions (a). The Stern–Völmer plot of I0/I versus the concentration of NP (b). The linear correlation between luminescent intensity and concentration of NP at low concentrations (c). Luminescent intensity of 1 after five recyclable experiments for the detection of NP in EtOH solution (d).
Moreover, the possible mechanism of luminescent quenching has been researched through the well matched PXRD patterns of CP 1 sample before and after immersion in the EtOH solution of NP, demonstrating that the framework of CP 1 keeps its integrity (Fig. S6). The possible reason for luminescence quenching may be a result of the strong electron-withdrawing ability rather than the collapse of the crystal structure due to the strongly electron-withdrawing ability of the nitro group [42, 49–52]. Otherwise, we attribute the quenching behavior to the interaction, such as π\( \cdots \)π interactions and H-bond interactions [53–55]. In brief, electron transfer and electrostatic interactions may contribute to the luminescence quenching process.
CONCLUSIONS
In summary, CP 1 was synthesized according to previous literature. Interestingly, the final 3D supramolecular structure of 1 is built from the 1D Zigzag chains through hydrogen-bonding interactions. In addition, CP 1 provides an excellent platform to study the detection of NP. Meanwhile, the detection limit of NP in EtOH is as low as 4.4 μM. Moreover, this compound shows good reusability after 5 cycles of sensing NP in EtOH solution.
REFERENCES
S. Y. Wu, H. Min, W. Shi, et al., Adv. Mater., 1805871 (2019). https://doi.org/10.1002/adma.201805871
Q. Y. Huang, Y. Zhao, and X. Wei, Russ. J. Inorg. Chem. 64, 592 (2019). https://doi.org/10.1134/S0036023619050085
O. P. Charkin, Russ. J. Inorg. Chem. 64, 615 (2019). https://doi.org/10.1134/S0036023619050048
H. Wang, W. P. Lustig, and J. Li, Chem. Soc. Rev. 47, 4729 (2018). https://doi.org/10.1039/c7cs00885f
J. Li, X. X. Wang, G. X. Zhao, et al., Chem. Soc. Rev. 47, 2322 (2018). https://doi.org/10.1039/C7CS00543A
W. P. Lustig, S. Mukherjee, N. D. Rudd, et al., Chem. Soc. Rev. 46, 3242 (2017). https://doi.org/10.1039/C6CS00930A
C. D. Si, D. C. Hu, Y. Fan, et al., Cryst. Growth Des. 15, 2419 (2015). https://doi.org/10.1021/acs.cgd.5b00205
C. Wang, D. M. Liu, and W. B. Lin, J. Am. Chem. Soc. 135, 13 222 (2013). https://doi.org/10.1021/ja308229p
K. Wang, Russ. J. Coord. Chem. 45, 371 (2019). https://doi.org/10.1134/S1070328419040092
D. Zhou, L. Lu, Y. Luo, et al., Russ. J. Coord. Chem. 44, (812) 2018, https://doi.org/10.1134/S1070328418120126
C. H. Zhang, Y. C. Liu, L. B. Sun, et al., Chem. Eur. J. 24, 2718 (2018). https://doi.org/10.1002/chem.201705399
Y. Rachuri, S. Subhagan, B. Parmar, et al., Dalton Trans. 47, 898 (2018). https://doi.org/10.1039/C7DT03667A
W. J. Ji, R. Q. Hao, W. W. Pei, et al., Dalton Trans. 47, 700 (2018). https://doi.org/10.1039/C7DT04113F
M. K. Wu, F. Y. Yi, Y. Fang, et al., Cryst. Growth Des. 17, 5458 (2017). https://doi.org/10.1021/acs.cgd.7b00984
H. M. Zhang, J. Yang, W. Q. Kan, et al., Cryst. Growth Des. 16, 265 (2016). https://doi.org/10.1021/acs.cgd.5b01226
B. H. Liu, D. X. Liu, K. Q. Yang, et al., Inorg. Chem. Commun. 90, 61 (2018). https://doi.org/10.1016/j.inoche.2018.02.008
Z. Wang, J. Yang, Y. S. Li, et al., Dalton Trans. 47, 5570 (2018). https://doi.org/10.1039/C8DT00569A
Z. J. Wang, L. J. Han, X. J. Gao, et al., Inorg. Chem. 57, 5232 (2018). https://doi.org/10.1021/acs.inorgchem.8b00272
L. F. Liang, L. Y. Liu, F. L. Jiang, et al., Inorg. Chem. 57, 4891 (2018). https://doi.org/10.1021/acs.inorgchem.7b03076
D. Zhou, L. Lu, Y. Luo, et al., Russ. J. Coord. Chem. 44, 439 (2018). https://doi.org/10.1134/S1070328418070060
A. Das, S. Jana, and A. Ghosh, Cryst. Growth Des. 18, 2335 (2018). https://doi.org/10.1021/acs.cgd.7b01752
B. Parmar, Y. Rachuri, K. K. Bisht, et al., Chem. Select. 1, 6308 (2016). https://doi.org/10.1002/slct.201601134
Y. Dai, H. J. Zhou, X. D. Song, et al., CrystEngComm 19, 2786 (2017). https://doi.org/10.1039/C7CE00236J
Z. Sun, Y. G. Li, Y. Ma, et al., Dyes Pigments 146, 263 (2017). https://doi.org/10.1016/j.dyepig.2017.07.015
L. Ma, Q. Zhang, H. Wu, et al., Eur. J. Inorg. Chem., 4221 (2017). https://doi.org/10.1002/ejic.201700874
X. M. Kang, R. R. Cheng, H. Xu, et al., Chem. Eur. J. 23, 13 289 (2017). https://doi.org/10.1002/chem.201702533
A. N. Au-Duong, and C. K. Lee, Cryst. Growth Des. 18, 356 (2018). https://doi.org/10.1021/acs.cgd.7b01360
D. Tian, X. J. Liu, R. Feng, et al., ACS Appl. Mater. Interfaces 10, 5618 (2018). https://doi.org/10.1021/acsami.7b15764
X. L. Zhang, Z. Y. Zhan, X. Y. Liang, et al., Dalton Trans. 47, 3272 (2018). https://doi.org/10.1039/C7DT02966G
J. C. Jin, J. Wu, G. P. Yang, et al., Chem. Commun. 52, 8475 (2016). https://doi.org/10.1039/C6CC03063G
R. F. Bogale, Y. Z. Chen, J. W. Ye, et al., New J. Chem. 41, 12 713 (2017). https://doi.org/10.1039/C7NJ02492D
X. Y. Guo, F. Zhao, J. J. Liu, et al., J. Mater. Chem. A 5, 20 035 (2017). https://doi.org/10.1039/C7TA05896A
X. Q. Wang, L. L. Zhang, J. Yang, et al., J. Mater. Chem. A 3, 12 777 (2015). https://doi.org/10.1039/C5TA00061K
D. S. Moore, Rev. Sci. Instrum. 75, 2499 (2004). https://doi.org/10.1063/1.1771493
Z. O. Tesfaldet, J. F. van Staden, R. I. Stefan, Talanta 64, 1189 (2004). https://doi.org/10.1016/j.talanta.2004.02.044
A. Ohashi, H. Ito, C. Kanai, et al., Talanta 65, 525 (2005). https://doi.org/10.1016/j.talanta.2004.07.018
S. R. Sushrutha, R. Hota, and S. Natarajan, Eur. J. Inorg. Chem. 2016, 2962 (2016). https://doi.org/10.1002/ejic.201600111
A. Buragohain, M. Yousufuddin, M. Sarma, et al., Cryst. Growth Des. 16, 842 (2016). https://doi.org/10.1021/acs.cgd.5b01427
Z. J. Wang, L. Qin, J. X. Chen, et al., Inorg. Chem. 55, 10 999 (2016). https://doi.org/10.1021/acs.inorgchem.6b01521
X. D. Zhu, Y. Li, W. X. Zhou, et al., CrystEngComm 18, 4530 (2016). https://doi.org/10.1039/C6CE00882H
G. M. Sheldrick, Acta Crystallogr., Sect. C 71, 3 (2015). https://doi.org/10.1107/S2053229614024218
Q. Zhao and C. D. Si, Cryst. Res. Technol. 54, 1 800 155 (2019). https://doi.org/10.1002/crat.201800155
X. Y. Xu and B. Yan, ACS Appl. Mater. Interfaces. 7, 721 (2015). https://doi.org/10.1021/am5070409
C. Liu and B. Yan, Photochem. Photobiol. Sci. 14, 1644 (2015). https://doi.org/10.1039/C5PP00107B
Y. L. Hu, M. L. Ding, X. Q. Liu, et al., Chem. Commun. 52, 5734 (2016). https://doi.org/10.1039/C6CC01597B
Z. Sun, M. Yang, Y. Ma, et al., Cryst. Growth Des. 17, 4326 (2017). https://doi.org/10.1021/acs.cgd.7b00638
X. L. Zhao, D. Tian, Q. Gao, et al., Dalton Trans. 45, 1040 (2016). https://doi.org/10.1039/C5DT03283K
H. Xu, C. S. Cao, and B. Zhao, Chem. Commun. 51, 10 280 (2015). https://doi.org/10.1039/C5CC02596F
Z. Sun, P. Hu, Y. Ma, et al., Dyes and Pigments 143, 10 (2017). https://doi.org/10.1016/j.dyepig.2017.04.015
Y. Peng, A. J. Zhang, M. Dong, et al., Chem. Commun. 47, 4505 (2011). https://doi.org/10.1039/C1CC10400D
Y. H. Huang, Q. L. Zhu, T. L. Sheng, et al., CrystEngComm 15, 3560 (2013). https://doi.org/10.1039/C3CE27020C
D. Y. Ma, W. X. Wang, Y. W. Li, et al., CrystEngComm 12, 4372 (2010). https://doi.org/10.1039/C0CE00135J
X. M. Lin, Y. J. Ding, S. M. Liang, et al., CrystEngComm 17, 3800 (2015). https://doi.org/10.1039/C5CE00478K
D. M. Wang, L. R. Zhang, G. H. Li, et al., RSC Adv. 5, 18 087 (2015). https://doi.org/10.1039/C4RA16599C
J. H. Cui, Z. Z. Lu, Y. Z. Li, et al., Chem. Commun. 48, 7967 (2012). https://doi.org/10.1039/C2CC34047J
ACKNOWLEDGMENTS
This work was supported by grants from the Natural Science Foundation of China (no. 21761030), the research projects of colleges and universities in Gansu provinc (no. 2017A-075), and Tianshui Normal University ‘QinglanTalents’ Project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No potential conflict of interest was reported by the authors.
Supplementary material
Rights and permissions
About this article
Cite this article
Peng Wang, Long, SJ. & Si, CD. A Photoluminescent Cd(II) Coordination Polymer with Highly Selective Detection for Nitrophenol. Russ. J. Inorg. Chem. 64, 1769–1774 (2019). https://doi.org/10.1134/S003602361914016X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602361914016X