Abstract
Extraction of lanthanum chloride and nitrate from neutral chloride and nitrate solutions with a binary extractant (R3NHA) based on an equimolar mixture of diglycolic acid N,N-dioctylamide and tri-n-octylamine was studied. It was shown that the extraction of lanthanum chloride with R3NHA proceeds by the mechanism of binary extraction of salts to give the LaA3 extractable compound in the organic phase. In the case of lanthanum nitrate extraction, complexes of a different composition are formed in the organic phase, with the ratio of the metal concentration in the organic phase to the initial concentration of the binary extractant being 1 : 1. The extractability of lanthanides in the system with this binary extractant increased with increasing atomic number of the metal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Lanthanides are widely used not only in the traditional branches of industry, but also in the modern manufacturing processes, as they possess luminescent, electronic, magnetic, and other useful properties. In most cases, this requires a high degree of purity of the lanthanide; however, separation of these metals is a challenge because of similarity of their chemical and physical properties. Among known techniques, extraction is one of the most efficient ways for lanthanide recovery, separation, and purification [1], and organophosphorus reagents, for example, di(2-ethylhexyl)phosphate and 2-ethylhexyl 2-ethylhexylphosphonate, are the most well-known commercial extractants [2]. However, organophosphorus extractants are toxic and pollute the environment, as their disposal gives phosphorus-containing wastes. Extractants based on carboxylic acid derivatives consisting only of C, H, O, and N atoms do not contain phosphorus and, from the environmental standpoint, they are promising for development of new processes for lanthanide extraction. The extraction of a number of lanthanides from nitrate and chloride solutions with mono-N,N-dioctylamide of diglycolic acid (HDGA) has been studied [3–5].

It was found that increasing pH of the aqueous phase leads to increasing distribution ratios of lanthanides, in conformity with the cation exchange mechanism of extraction. Unlike known extractants, carboxylic acid derivatives containing only C, H, and O atoms (e.g., Versatic 10), which extract lanthanides from neutral media [6], HDGA provides almost quantitative recovery of lanthanides from weakly acidic solutions (1 ≤ pH ≤ 3); i.e., efficiency of extraction with HDGA is comparable with the extraction with organophosphorus compounds. The extraction stoichiometry in HDGA (HA) systems was determined; it was found that lanthanides are recovered from nitrate solutions to give LnA3 complexes [3, 4], while extraction from chloride media may also give, for example, in the case of erbium, LnA2Cl compounds [5].
Apart from traditional extractants, binary extractants that can also be classified as ionic liquids (ILs), that is, organic salts existing in the liquid state at room temperature, are used for the recovery and separation of lanthanides [7–17]. Extraction systems using ILs based on diglycolic acid N,N-dioctylamide and quaternary ammonium bases were reported [18–20]. It was shown [18, 19] that phase distribution of lanthanide nitrates in systems containing the IL representing the salt of HDGA and methyltrioctylammonium (R4NA) differs from the phase distribution observed in the extraction of these metals with a mixture of the initial extractants. According to [19], the extraction of europium nitrate with R4NA solutions in dodecane results in the formation of Eu(NO3)3 · (R4NA)3 solvates. An extractable complex of a similar composition is formed upon the extraction of neodymium from nitrate solutions with dodecane solutions of R4NA [19]. However, with ionic liquid, methyltrioctylammonium nitrate, as the solvent and NaNO3 as the salting-out agent, the stoichiometry of extraction changes and neodymium migrates to the organic phase as Nd(NO3)3 · R4NA [18]. As the concentration of NaNO3 increases, the distribution ratios of lanthanides increase and at very high concentrations of nitrate ions, the extraction of metals with ionic liquid proceeds by anion exchange and coordination mechanisms [18, 19].
It is noted that ILs based on HDGA have a higher extraction capacity than a mixture of initial extractants at the same equilibrium pH values [18]. It appeared of interest to study the extraction of lanthanide salts from nitrate and chloride solutions with a new binary extractant based on HDGA and tri-n-octylamine (TOA).
EXPERIMENTAL
The initial solutions of lanthanum nitrate and chloride were prepared by dissolving weighed portions of La(NO3)3 · 6H2O (reagent grade) and LaCl3 · 7H2O (reagent grade) in distilled water. The solutions of Nd, Eu, and Er nitrates were prepared by dissolving weighed portions of reagent grade metal oxides in concentrated HNO3 followed by repeated evaporation of aqueous solutions on a water bath to remove excess acid. The extractant, diglycolic acid N,N-dioctylamide, was synthesized by a reported procedure [3]. The binary extractant, TOA–HDGA, was prepared by dissolution of equimolar amounts of tri-n-octylamine (Fluka) and N,N-dioctylamide of diglycolic acid in toluene.
The phases were stirred at 20°C in test tubes with ground-glass joints for 15 min; this was sufficient to attain invariable distribution ratios of lanthanides.
The lanthanide concentrations in the initial solutions and aqueous phases after extraction were determined by titration with Trilon B in the presence of xylenol orange. The concentrations of lanthanides in organic phases were determined as the difference between the concentrations in the initial solution and aqueous phase after extraction.
RESULTS AND DISCUSSION
The extraction of lanthanides with a binary extractant based on TOA and HDGA (R3NHA) was studied in relation to lanthanum salts. The stoichiometry of phase distribution was established by measuring the isotherms of extraction of lanthanum chloride from 0.5 M solutions of NaCl and lanthanum nitrate from aqueous solutions without addition of the salting-out agent and from 0.5 M solutions of NaNO3 into a 0.01 M solution of R3NHA in toluene (Fig. 1). The data presented in Fig. 1 (curve 1) indicate that in the extraction of LaCl3 from chloride solutions with saturation of the organic phase, the extractant to metal ratio was close to 3, which attested to the formation of the LaA3 extractable salt (A– is the HDGA anion). Presumably, in the general case without considering the possible reactions between the components in the organic phase, lanthanum chloride distribution in the R3NHA-containing system is described by the equation of the binary extraction of salts:
The isotherm of La(NO3)3 extraction from aqueous solutions without a salting-out agent (Fig. 1, curve 2) has a flat section at a 1 : 1 ratio of the metal concentration in the organic phase to the initial concentration of the binary extractant. Presumably, compounds containing metal-containing anions with mixed ligands are formed in the organic phase in the R3NHA system, as takes place, for example, in the extraction of lanthanide nitrates with methyltrioctylammonium dialkylphosphinate [15]. The formation of compounds of this composition can be attributed to more potent complexing properties of nitrate ions compared with chloride ions and to lower hydration energy of \({\text{NO}}_{3}^{-}\) in the aqueous phase. The extraction of lanthanum nitrate with the binary extractant is described, in this case, by the equation that implies two extractable compounds
It is known that the extraction of lanthanides from nitrate solutions with trioctylammonium nitrates involves the formation of extractable compounds with metal-containing anions [21]; therefore, extraction with formation of one extractable compound can also be assumed:
In parallel with binary extraction at high nitrate concentrations, solvates can be formed in the aqueous phase according to the equation
With excess extractant, the extractable compounds can be solvated by the binary extractant or trioctylammonium nitrate molecules.
The extraction of lanthanum nitrate is more efficient from nitrate solutions (Fig. 1, curve 3) than from aqueous solutions without a salting out agent and, the more so, it is more efficient than extraction of lanthanum chloride. Under saturation conditions, the ratio of the initial extractant concentration to the concentration of lanthanum in the organic phase is equal to unity, like in the case of extraction from solutions without a salting-out agent.
The effect of NaCl and NaNO3 concentration on the extraction of lanthanum chloride and nitrate, respectively, was studied (Fig. 2). It follows from the data of Fig. 2 (curve 1) that an increase in the NaCl concentration is accompanied by a minor increase in the distribution ratios of lanthanum chloride according to equation (1). An increase in the NaNO3 concentration leads to considerably higher recovery of lanthanum nitrate (Fig. 2, curve 2), which also confirms the co-extraction of nitrate ions with lanthanum and their presence in the extractable complex according to equations (2)–(4).
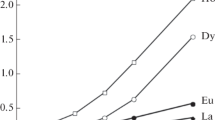
The extraction of lanthanide nitrates from aqueous solutions in the absence of NaNO3 and in the presence of 0.5 M NaNO3 with a 0.01 M solution of R3NHA in toluene was compared (Fig. 3). It can be seen from the obtained results that extratability of lanthanides increases with increasing atomic number of the metal; this is also characteristic of binary extractant systems based on dialkyl phosphoric [11, 12] and dialkylphosphinic acids [13, 14].
The studies demonstrated high capacity of the TOA–HDGA binary extractant with respect to lanthanide nitrates, which may have practical importance. In addition, in the systems with this binary extractant under the experimental conditions, no precipitates were formed (CLn(o) ≤ 0.01 mol/L), whereas for the extraction with binary extractants based on dialkyl phosohoric acids, the solubility of extractable compounds does not exceed 0.006 mol/L [11, 12]. The results show good prospects for extraction systems based on TOA–HDGA to be used, for example, in liquid chromatography and in combined extraction and chromatographic separation processes [22–25]. The binary extractant based on TOA and HDGA could also find use in extraction processes using liquid membrane principle [26–30]. This is due to the fact that these processes do not require large amounts of extractants.
REFERENCES
F. Xie, T. A. Zhang, D. Dreisinger, et al., Miner. Eng. 56, 10 (2014). https://doi.org/10.1016/j.mineng.2013.10.021
X. L. Wang, W. Li, S. L. Meng, et al., J. Chem. Technol. Bio. 81, 761 (2006). https://doi.org/10.1002/jctb.1532
K. Shimojo, H. Naganawa, J. Noro, et al., Anal. Sci. 23, 1427 (2007). https://doi.org/10.2116/analsci.23.1427
K. Shimojo, N. Aoyagi, T. Saito, et al., Anal. Sci. 30, 263 (2014). https://doi.org/10.2116/analsci.30.263
R. Safarbali, M. R. Yaftian, and A. Zamani, J. Rare Earths 34, 91 (2016). https://doi.org/10.1016/S1002-0721(14)60583-4
F. Kubota, K. Shinohara, K. Shimojo, et al., Sep. Purif. Technol. 24, 93 (2001). https://doi.org/10.1016/S1383-5866(00)00215-X
X. Sun, Y. Ji, F. Hu, et al., Talanta 81, 1877 (2010). https://doi.org/10.1016/j.talanta.2010.03.041
H. L. Yang, W. Wang, H. M. Cui, et al., Chin. J. Anal. Chem. 39, 1561 (2011). https://doi.org/10.1016/S1872-2040(10)60475-6
L. Guo, J. Chen, L. Shen, et al., ACS Sust. Chem. Eng. 2, 1968 (2014). https://doi.org/10.1021/sc400541b
F. Kubota, Y. Baba, and M. Goto, Solv. Extr. Res. Dev. Jpn. 19, 17 (2012). https://doi.org/10.15261/serdj.19.17
S. N. Kalyakin, V. I. Kuz’min, and M. A. Mulagaleeva, Tsvetn. Met., No. 3, 51 (2011).
S. N. Kalyakin, V. I. Kuzmin, and M. A. Mulagaleeva, Hydrometallurgy 151, 116 (2015). https://doi.org/10.1016/j.hydromet.2014.11.013
N. S. Egorova, V. V. Belova, A. A. Voshkin, et al., Russ. J. Inorg. Chem. 50, 1781 (2005).
V. V. Belova, A. A. Voshkin, A. I. Kholkin, and A. K. Payrtman, Hydrometallurgy 97, 198 (2009). https://doi.org/10.1016/j.hydromet.2009.03.004
V. V. Belova, A. A. Voshkin, N. S. Egorova, and A. I. Kholkin, Russ. J. Inorg. Chem. 55, 629 (2010). https://doi.org/10.1134/S0036023610040224
Yu. A. Zakhodyaeva, V. V. Belova, N. S. Egorova, and A. I. Khol’kin, Khim. Tekhnol. 16, 23 (2015).
V. V. Belova, Yu. A. Zakhodyaeva, and A. I. Kholkin, Russ. J. Inorg. Chem. 60, 526 (2015). https://doi.org/10.1134/S0036023615040026
A. Rout and K. Binnemans, Ind. Eng. Chem. Res. 53, 6500 (2014). https://doi.org/10.1021/ie404340p
A. Rout, K. A. Venkatesan, T. G. Srinivasan, and P. R. Vasudeva Rao, Sep. Purif. Technol. 95, 26 (2012). https://doi.org/10.1016/j.seppur.2012.04.020
L. Qiu, Y. Pan, W. Zhang, and A. Gong, PLoS ONE 13, 1 (2018). https://doi.org/10.1371/journal.pone.0201405
A. Sahoo, J. Swain, and B. C. Bhatta, OSR J. Appl. Chem. 10, 52 (2017). https://doi.org/10.9790/5736-1010015258
A. Kostanyan, M. Martynova, A. Erastov, and V. Belova, J. Chromatogr. A 1560, 26 (2018). https://doi.org/10.1016/j.chroma.2018.05.032
A. Kostanyan and A. Erastov, J. Chromatogr. A 1572, 212 (2018). https://doi.org/10.1016/j.chroma.2018.08.039
A. E. Kostanyan and A. A. Erastov, J. Chromatogr. A 1406, 118 (2015). https://doi.org/10.1016/j.chroma.2015.05.074
A. E. Kostanyan, A. A. Erastov, and O. N. Shishilov, J. Chromatogr. A 1347, 87 (2014). https://doi.org/10.1016/j.chroma.2014.04.064
V. V. Belova, A. E. Kostanyan, Y. A. Zakhodyaeva, et al., Hydrometallurgy 150, 144 (2014). https://doi.org/10.1016/j.hydromet.2014.10.011
A. E. Kostanyan, Russ. J. Inorg. Chem. 63, 287 (2018). https://doi.org/10.1134/S0036023618020122
A. E. Kostanyan, A. M. Safiulina, I. G. Tananaev, and B. F. Myasoedov, Dokl. Chem. 404, 203 (2005).
A. E. Kostanyan, A. M. Safiulina, and I. G. Tananaev, Khim. Tekhnol. 7 (8), 35 (2006).
A. E. Kostanyan, Khim. Tekhnol. 13 (6), 376 (2012).
Funding
A part of the study was carried out within the state assignment of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, and Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, in the field of fundamental research and was supported by the Russian Foundation for Basic Research (project nos. 17-03-00263 and 18-29-24069).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Belova, V.V., Martynova, M.M., Baulin, V.E. et al. Extraction of Lanthanides with a Binary Extractant Based on Diglycolic Acid N,N-Dioctylamide and Trioctylamine. Russ. J. Inorg. Chem. 64, 1059–1062 (2019). https://doi.org/10.1134/S0036023619080023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619080023