Abstract
Wedgefish (family Rhinidae) is a group of elasmobranchs that experience a global threat due to its highly valued fins. Similar condition happens to most species of wedgefish inhabiting Indonesian waters where fishing activities are intense without sufficient management controls and lack of supporting studies on their sustainabilities. In order to get a picture of wedgefish populations in Indonesia, the current study employing demographic analysis was performed to know the population status of two wedgefish species (Rhynchobatus australiae and Rhina ancylostoma) from western Indonesian waters (including the Java Sea, Karimata and southern Makassar Straits). Age-based matrix models were used involving two scenarios of populations with and without fishing. Monte carlo simulation was applied to incorporate uncertainties in life-history parameters. The results show contrasting productivities for R. australiae and R. ancylostoma. R. australiae is sufficiently productive, indicated by high population growth rates in both with and without fishing scenarios. In contrast, the population of R. ancylostoma grows positively only in the unfished scenario, but the growth is negative in the with-fishing scenario. This finding indicates that the current level of exploitation caused the depletion in the population of R. ancylostoma. The current level of fishing can be maintained for R. australiae to give optimum benefits to fishery communities, while for R. ancylostoma, substantial reduction in fishing is required. Protection of young fish (juveniles up to age at first reproduction) is recommended in both fish since population growths are very sensitive to the changes in these stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Elasmobranchs (sharks, rays and skates) are considered one among the most endangered marine biota (Campana et al., 2017). Their intrinsic biological traits make them vulnerable to exploitation compared to teleost fishes (Hoenig and Gruber, 1990; Stobutzki et al., 2002; Blaber et al., 2009). Consequently, the sustainability of elasmobranchs has become a global concern in recent years (Garcia et al., 2008, Kynoch et al., 2015; Ward-Paige, 2017, Braccini et al., 2020).
Order Rhinopristiformes (including wedgefish) is arguably the most threatened elasmobranch globally (Dulvy et al., 2014; Jabado, 2018). This group is claimed to be experiencing global declines due to overexploitation (Jabado, 2018; D’Alberto et al., 2019). Moore (2017) noted local extinctions had been reported for some species of guitarfish (probably including wedgefish). The very high-value fins of sawfish and guitarfish become the driver of this threat (Moore, 2017; Kyne et al., 2020). All species in wedgefish (family Rhinidae) except for Rhynchobatus palpebratus, are currently listed as Critically Endangered in IUCN Red List (The IUCN Red List of Threatened Species: https://www.iucnredlist.org. Version 03/2020). Furthermore, at the CoP 18, CITES also decided to list all species of wedgefish in Appendix II.
Among all wedgefish species, Rhynchobatus australiae and Rhina ancylostoma are commonly caught in Indonesian waters. Both species are demersal and inhabit coastal areas to a depth of about 60 m (Last et al., 2016). Almost all body parts of wedgefish are utilized, particularly their fins that have the highest price in international market (Djunaidi, PESIHIPINDO, Surabaya, Indonesia, personal communication, 2020).
As the largest elasmobranch-exploiting country (Dharmadi et al., 2009; Fahmi et al., 2013; Tull, 2014) and is located in Indo-West Pacific which is the center of wedgefish diversity (Kyne et al., 2020), Indonesia is required to implement sustainable management strategy towards the wedgefish. Unfortunately, the population status of these wedgefish in Indonesia remains unknown. This situation makes the research on the population status of wedgefish in Indonesian waters urgent.
Despite the global concern about these fish, to the best of our knowledge, so far, no stock assessment research has been conducted for these two wedgefish species both in Indonesia and other regions. D’Alberto et al. (2019) did a demographic analysis on Rhinopristiformes which includes R. australiae, while Kyne et al. (2020) did extinction risk study on wedgefish and giant guitarfish including both R. australiae and R. ancylostoma. However, their works were aimed to evaluate “the biological nature of populations” rather than to assess particular stocks which are subject to fishing. We conducted the present study to assess the population status of R. australiae and R. ancylostoma in western Indonesian inner waters. A demographic model using Leslie projection matrix was applied to achieve this goal. Two scenarios of populations with and without fishing were incorporated, hence, complementing the analysis done by D’Alberto et al. (2019) for R. australiae.
The demographic model was chosen for the following reasons. Firstly, this is a standard method applied to elasmobranch (Hoenig and Gruber, 1990; Simpfendorfer, 2005; Gedamke et al., 2007). Secondly, this method does not require extensive data as a full age-structured stock assessment does (Tsai et al., 2010; Hisano et al., 2011). Therefore, this method is suitable for fisheries in Indonesia which are mostly data-poor, in particular elasmobranch fisheries. This study is expected to generate scientific information which can be used as input to design suitable management strategies in attempts to achieve fisheries sustainability of these wedgefish in Indonesia.
MATERIALS AND METHODS
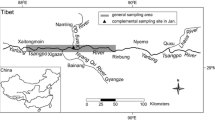
The current study applied demographic analysis to assess the population status of R. australiae and R. ancylostoma in western Indonesian waters which includes Karimata Strait (Fisheries Management Area; FMA 711), Java Sea (FMA 712) and southern Makassar Strait (FMA 713) as shown in Fig. 1. Leslie projection (age-based) matrix model was chosen, rather than the stage-based model, since the former is more suitable for long-lived species like elasmobranch (Mollet and Cailliet, 2002). We applied scenarios of population with and without fishing to find the effect of current fishing mortalities to the sustainabilities of both wedgefish species. All analyses were done in R Language and Environment for Statistical Computing (R Core Team, 2019).
The basic equation of the matrix model was described by Simpfendorfer (2005) and Geng and Zhu (2017):
where Nt the vector representing age structure of the fish population at time t and A the Leslie projection matrix such that:
Na,t represents the number of individuals of age a at year t, fa fecundity (female pups) at age a, Sa survival rate from age a to a+1. To apply this model, information on life-history parameters of fish of interest are required. These include parameters of the von Bertalanffy growth model (\({{L}_{\infty }}\), k, L0), age at first reproduction (ap), fecundity at age a (fa), longevity or maximum age (amax), and frequency of reproduction or reproductive cycle (RC). Information on natural and fishing mortality rates at age (Ma and Fa) are also needed to know survival rates for each age (Sa).
Nt and Nt+1 were assumed to be stable which could be represented by eigenvectors of the matrix A with the associated leading eigenvalue λ representing the finite rate of population increase. From this, the associated intrinsic rate of population increase (r) could also be obtained by equality \(r = \ln {\kern 1pt} {{\lambda \;}}\). Also, other parameters borrowed from life table analysis i.e. net reproductive rates (R0) and doubling time (t×2) for cases λ > 1 were also derived (\({{R}_{0}} = \sum\nolimits_{a = 0}^{{{a}_{{{\text{max}}}}}} {{{S}_{a}}{{f}_{a}}} \) and \({{t}_{{ \times 2}}} = \frac{{\ln {\kern 1pt} 2}}{r}\)). In addition, for cases where λ < 1, collapse time (tcoll) defined as the time by which population decrease to less than 1% of the initial abundance, was also computed \(\left( {{{t}_{{{\text{coll}}}}} = \frac{{\ln {\kern 1pt} 0.01}}{r}} \right)\).
The primary data we had in this study was length frequency data of R. australiae and R. ancylostoma from sampling programs in 2017–2019 at several fish landing ports i.e Tegalsari Coastal Fishing Port—Tegal, Bajomulyo Coastal Fishing Port—Pati, Tasik Agung Coastal Fishing Port—Rembang, Brondong Archipelagic Fishing Port—Lamongan and and Sungai Kakap Fish Landing Port—Pontianak (Fig. 1) from which fishermen operate in Java Sea, Karimata and southern Makassar Strait. This data was used to estimate total mortality rates (Z) of each species. We also had length-frequency data from the eastern Indian Ocean (southern waters of Java Island, FMA 573), which, despite not being used in estimating Z, was also useful in this study. The rest of life history data was obtained directly or indirectly from existing literature all around the world.
Having length-frequency data, usually, von Bertalanffy growth parameters can be obtained from length-based methods like Electronic Length Frequency Analysis/ELEFAN (Pauly and David, 1981; Taylor and Mildenberger, 2017; Wang et al., 2020). The trials to implement ELEFAN method for both R. australiae and R. ancylostoma were really attempted, making use of package TropFishR (Mildenberger et al., 2017). Unfortunately, too few fish individuals caught daily hindered the ELEFAN method to work reliably. So, other methods including citing literature were attempted to obtain growth parameters.
Rhynchobatus australiae
White et al. (2014) have done age and growth study for species complex Rhynchobatus spp. which included R. australiae, R. laevis and R. palpebratus in eastern Australia. D’Alberto et al. (2019) re-examined their data and suggested that they comprised primarily R. australiae. Ideally, this study could directly use the growth parameters generated by White et al. (2014). However, further examination showed that their result did not match our length-frequency data (see Results and Discussions). So, we re-analyzed the von Bertalanffy growth model ourselves, partly by reconstructing age-length keys from White et al. (2014). Two parameters, asymptotic length (\({{L}_{\infty }}\)) and length at birth (L0) were predetermined without age−length data. \({{L}_{\infty }}\) was estimated through Froese and Binohlan (2000) formula using maximum total length (TL) observed in our data (323 cm). For L0, Weigmann (2011) suggested between 46−50 cm TL. White et al. (2014) used L0 = 50 cm in their analysis. However, our data showed the minimum size of fish caught to be 39 cm, lower than those suggested lengths at birth. So, in this work L0 was taken as the midpoint of 39 and 50 cm TL. After that, the parameter of somatic growth rate (k) was estimated by fitting nonlinear regression to age-length data from White et al. (2014) using the package minpack.lm (Elzhov et al., 2016).
Age at first reproduction was set to be the age at first pupping (ap); ap was derived from age at maturity (am), which, in turn, was derived from the length at maturity (Lm). Weigmann (2011) suggested Lm for females to be 155 cm TL. While D’Alberto et al. (2019), in their analysis, took Lm = 150 cm, borrowing from Rhynchobatus djiddensis. We did not take into account the estimated Lm = 280 cm from White and Dharmadi (2007) since it was unreasonably too close to \({{L}_{\infty }}\). Using the inverse von Bertalanffy function, the two Lm from Weigmann (2011) and D’Alberto et al. (2019) were converted to tm = 5.4 and 5.7 years. For the analysis of the matrix model, 1 year lag for gestation was assumed. So, ap was estimated to be between 6.4−6.7. Since the resulting ap was fractional, while the matrix model required round numbers, ap was set to be 6−7 years. This agrees with the assumed age at reproduction used by White et al. (2014) i.e 6 years.
White and Dharmadi (2007) identified the litter size/fecundity (ftot) for this species ranging between 7−19 pups per individual mother with mode 14 pups. No information about the relationship between mother’s age and litter size, so constant fecundity across age (fa = f) was assumed. Furthermore, since no information on sex ratio of litter was found, it was set to be 1:1, agreeing with D’Alberto et al. (2019). So, the number of female offspring (f) equals half the total fecundity (ftot).
Rhina Ancylostoma
Similar to R. australiae, \({{L}_{\infty }}\) parameter for R. ancylostoma was estimated using Froese and Binohlan (2000) formula, and L0 was obtained from combining the minimum size in our data and information from the literature. Our data showed the minimum size observed to be 43 cm TL. Meanwhile, Last et al. (2016) suggested the size at birth to be 46−48 cm. Given these, we set L0 as the midpoint of 43 and 48 cm. One more growth parameter k could not be determined since no relevant information was found. However, considering the usual range of k in elasmobranch (Cortés, 2000; Frisk et al., 2001) and the k value for R. australiae found in this study, it was reasonable to suggest the true k value for R. ancylostoma is in the interval (0.08, 0.20). Therefore, four equidistant points of k (0.08, 0.12, 0.16 and 0.20) were chosen from this interval, and further analysis was attempted for each of these k values.
No much information is available regarding age at first pupping (ap) for R. ancylostoma. The only information is the estimate of Lm by Last et al. (2016) i.e. 180 cm, which is ambiguous between length at first maturity or length at first pupping. However, this value is still useful to estimate age at first pupping (ap). Given multiple k, then multiple estimates of am and accordingly ap were obtained. Each k has one am which was then converted to ap (ap = am + 1). From the lowest to highest k, we got ap = 11.4, 7.9, 6.2, and 5.2 years. To round up these numbers, at the same time incorporating ambiguity of Lm from Last et al. (2016), then ap was set to be 10−12, 6−8, 5−7 and 5−6 years, in which the last ap (5−6 instead of 4−6 years) was chosen because ap = 4 years was considered to be too early.
Several authors studied fecundity of R. ancylostoma. Masuda et al. (1975) suggested total fecundity (ftot) to be four pups per mother, Last and Stevens (2009) suggested nine pups and Devadoss and Batcha (1995) 7−9 pups. However, the broadest range of ftot is identified by Raje (2006) i.e., between 2−11 pups. Also, there does not seem to be a relationship between mother’s age and litter sizes. So, ftot was determined to be 2−11 with mode 8 pups. Again, litter sex ratio 1:1 was assumed.
For both R. australiae and R. ancylostoma, there is no information about longevity. So, longevities/maximum ages (amax) were determined theoretically by means of Taylor’s (1958) equation and Fabens’ equation (used by: Goldman et al., 2006; Kadri et al., 2014). The two methods generated different estimates. Moreover, in R. ancylostoma, multiple k values resulted in multiple amax. So, in total there were 8 amax estimates for R. ancylostoma, two for each scenario of k.
Total mortality rates (Z) of both species were estimated using length-based linearized catch curve, making use of length-frequency data available. The formula used in the regression follows Sparre and Venema (1998):
where \(C\left( {{{L}_{1}},{{L}_{2}}} \right)\) is the number of individuals caught with the length between L1 and L2, \(\Delta a\) is age increment for fish from length L1 to reach L2, \({\text{a}}\left( {\frac{{{{L}_{1}} + {{L}_{2}}}}{2}} \right)\) is the age at length \(\frac{{{{L}_{1}} + {{L}_{2}}}}{2}\). This method assumes constant Z with respect to time (resulting in Za = Z), which is unfortunate given fluctuating fishing efforts toward both fish. However, this is the best we can do in the face of limited data. Data shortages also pushed to pool both sexes (male and female) in generating single estimates of Z. Which points were included in the regression were assessed visually considering whether or not particular ages had been fully selected by fishing gear. From the linearized catch curve, selectivity at age (Sa) was also derived. In this study, gear selectivity was assumed to be logistic, which is reasonable considering that sharks and rays in this region were mostly caught by cantrang (seine net). Total mortality and selectivity analyses were implemented using the TropFishR package (Mildenberger et al., 2017).
As constant Z were used, constant natural mortality rates (Ma = M) were also assumed in this study. Several methods were applied to estimate these constant M, i.e. Alverson and Carney’s (1975) method, two methods of Hoenig’s (1983) i.e for fish and for a combination of mollusks, fish and cetaceans, Pauly’s (1980) method and two methods by Then et al. (2015), i.e third and sixth methods which use amax and k. All computations of M were automated in package TropFishR (Mildenberger et al., 2017).
No study has documented the RC for either R. australiae or R. ancylostoma. D’Alberto et al. (2019) simply assumed 1 year RC for R. australiae without justification. To extend the generality of RC, this study accounted for one year and two years RC for both R. australiae and R. ancylostoma, following Geng and Zhu (2017) for the case of blue shark (Prionace glauca).
After all life-history parameters were readily available, matrix models were computed for each species, for each RC, for each scenario of fished and unfished populations and additionally for each k in R. ancylostoma. As some parameters have multiple (uncertain) values, these uncertainties were accounted for by means of Monte Carlo simulation. Matrices were generated 10000 times, with each time single random values of each life history parameter were used (Cortés, 2002; Geng and Zhu, 2017). For this purpose, probability distribution for parameters ap, ftot, amax, M and Z were constructed based on the ranges and modes found; ap and amax were set to be discrete-uniformly distributed with parameters the lower and upper bounds of each; ftot was set to be triangularly distributed with parameters the lowest, highest, and mode of total fecundity. M also followed triangular distribution with parameters the lowest, highest, and average of natural mortality estimates. Z was assumed to be normally distributed with parameters the estimate of Z itself and standard error from regression in the linearized catch curve. The complete list of parameters used in matrix models is summarized in Tables 1, 2.
Each iteration generates one matrix A along with the corresponding λ, r, t×2, (or tcoll) parameters. So, each parameter has 10000 samples in each scenario. From these, the median and 95% confidence interval (CI) of each output parameter were calculated.
After that, 10000 R0 was computed separately, also making use of random samples of life-history parameters. Accordingly, the median and 95% CI for R0 were derived.
To demonstrate more clearly the long term trajectories of populations in each scenario, ten years projections were constructed. For this, the normal distribution for λ were assumed with the parameters the mean and standard deviation of λ samples from the matrix model. Each year, one random λ was drawn, and using this, 5000 samples of the female population at time t (Nt) were generated. From every 5000 Nt samples, the mean and 95% CI were computed.
To find the relative contribution of age, survival, and fecundity to the population growth rate (λ or r), then elasticity analysis was conducted. One matrix A was generated by drawing one random value for each of ap, ftot, amax, M and Z from their distributions. Leading eigenvalue and the associated eigenvector of matrix A were then computed. Elasticities were calculated using the formula (Simpfendorfer, 2005):
where aij elements of projection matrix A, v and w are left and right eigenvectors of A, and \(\langle v,w\rangle \) is the dot product of both eigenvectors. The elasticity of each age, the elasticity of survival and fecundity were simply derived from summing the relevant elements eij.
RESULTS AND DISCUSSIONS
Wedgefish Fisheries in Western Indonesian Inner Waters
In the western Indonesian inner waters, four species of wedgefish i.e Rhynchobatus australiae, R. springeri, R. laevis and Rhina ancylostoma, are commonly caught. These fish are caught both as target and bycatch. Fishers targetting wedgefish usually use tangle net as fishing gear, which is locally known as jaring liongbun or jaring kemejan (Sadri and Yuneni, 2019). As bycatch, wedgefish are caught by cantrang (kind of seine net), bottom longline, bottom gillnet and hand line (Sadri and Yuneni, 2019; Yuwandana et al., 2020). The latter four gears target demersal teleost fishes, but wedgefish are also caught. Most of wedgefish caught in these waters are bycatch from cantrang.
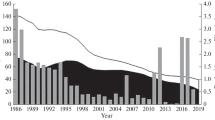
In Indonesia, there is no official data on species-specific catch/production of wedgefish. Until 2016, data on annual production of mixed wedgefish per FMA was documented by Ministry of Marine Affairs and Fisheries (MMAF). However, in 2016 MMAF changed its data collection system to one data. Since then, wedgefish and other rays have been reported as just “rays”. DGCF-MMAF (2017) reported the annual production of wedgefish for each FMA in Indonesia. Despite the category “whitespotted wedgefish” used by DGCF-MMAF, it is actually a mixture of all different wedgefish species. Figure 2 shows the annual production of wedgefish in western Indonesian inner waters during 2005−2016, pooled from FMA 711–713 according to DGCF-MMAF (2017). Figure 2 indicates that the catch of wedgefish has been declining, particularly from 2005 to 2008. However, the official data is usually considered not to represent the actual production. This is because of several problems in catch recording, i.e insufficient number of officers collecting the data, double-counting issues between subnational and national level, misidentification and other errors. Notwithstanding, Simeon et al. (2019) suggested the decrease in the number of vessels catching wedgefish, has contributed to the decline in wedgefish catch.
Length Frequency Distribution
Sampling programs in 2017−2019 around western Indonesian inner waters recorded a total of 2064 individuals of R. australiae and 334 of R. ancylostoma. The sizes of fish ranged between 39−300 cm TL for R. australiae and 43−297 cm TL for R. ancylostoma. It was this length-frequency data which was then used in the estimation of total mortality rates (Z). The length-frequency distributions for both wedgefish are displayed in histograms in Fig. 3.
The raw data contained one individual of R. australiae with TL 19 cm. However, this value is doubtful. There was no other fish with a length close to this both from the western Indonesian inner waters and from the eastern Indian Ocean. The second smallest individual was 39 cm, which was too far from 19 cm. Moreover, Weigmann (2011) found size at birth for R. australiae is between 46−50 cm. So, in this study 39 cm was chosen as the minimum length of R. australiae caught which was confirmed by the existence of individuals with similar sizes.
The maximum length observed from the eastern Indian Ocean was 323 cm, higher than the one from western Indonesian inner waters (300 cm). Considering the proximity of the two regions, the maximum size from the eastern Indian Ocean was used in generating asymptotic length. Actually, our data recorded an individual with TL 401 cm from the eastern Indian Ocean. However, with the similar reason as the minimum length, we set the second largest TL, 323 cm, as the maximum length naturally occurring for R. australiae. Those extreme small and large values (19 and 401 cm) were likely technical mistakes in the recording by enumerators. Fish of size 19 cm, if it really existed, was likely to be an embryo that was accidentally accounted for in the measurement.
For R. ancylostoma, the minimum and maximum sizes observed from western Indonesian inner waters were 43 and 297 cm TL. These values were used solely in the analysis because these were the smallest and largest lengths observed in the whole data. Furthermore, there were other lengths at the proximity of these values, confirming their validity.
Currently, the maximum size of 323 cm TL for R. australiae may be the highest ever recorded. White and Dharmadi (2007) reported the maximum size of 300 cm TL in eastern Indonesia, and White et al. (2014) observed only 263 cm TL in Australia. Meanwhile, for R. ancylostoma Vidthayanon (2005) in Borrell et al. (2011) suggested it attains as long as 300 cm in Thailand, higher than recorded in this study. However, in Indonesia, the TL of 297 cm might be the highest. Other studies in Indonesia reported the maximum size of only 270 cm TL (White et al., 2006; Last et al., 2016) and 250 cm TL (White and Dharmadi, 2007).
The length-frequency distribution for R. australiae is right-skewed (left-truncated), while that of R. ancylostoma is more symmetric (Fig. 3). From the perspective of conservation, catch with left-truncated size distribution is bad, since this is an indication of non-selective fishing gears. Young fish of R. australiae are as susceptible as adult fish to fishing, and this is generally not desired. On the other hand, more symmetric length-frequency means young R. ancylostoma is not fully selected, so it has higher probabilities to survive the fishing than adult fish.
From the perspective of analysis, however, samples with right-skewed length distribution are preferred. This gives more confidence about the larger part of size frequency in the catch proportionately resembling the frequency in nature. The decreasing frequency to the right of the highest-frequency length class seems to be solely made by mortality. It is what is assumed by logistic selectivity as used in ELEFAN method (Mildenberger et al., 2017). Symmetric length frequency distribution, on the other hand, raises suspicion of selectivity playing a role in shaping the right tail of length-frequency as it does to the left tail. This, in turn, raises doubt about the validity of the logistic-selectivity assumption. Another thing to notice is the small number of catches of R. ancylostoma. The fish is six times more rarely caught than R. australiae. The poor sample size of R. ancylostoma might also cause bias in its length frequency distribution (Fig. 3b). Whether it is selectivity assumption not met or insufficient samples, all can lead to biased conclusions of this study. However, currently, this is the best available data, hence, further analysis relies on this data.
Growth Models
Re-analysis of von Bertalanffy growth model for R. australiae produced \({{L}_{\infty }}\) = 307.9 cm, k = 0.095/year as displayed in Table 1 and plotted in Fig. 4a. These values are quite different from the ones estimated by White et al. (2014) i.e \(~{{L}_{\infty }}\) = 256.630 cm TL and k = 0.40/year for two parameters von Bertalanffy model and \({{L}_{\infty }}\) between 204.547−257.132 cm TL for all growth models they attempted. Those\({{\;\;}}{{L}_{\infty }}\) are suspicious because our data shows maximum length observed is 323 cm TL and there are a number of fish larger than 260 cm TL. While \({{L}_{\infty }}\) can be lower than the maximum observed length, in this case, the one estimated by White et al. (2014) is too low in contrast with our data. Furthermore, based on data on 230 shark stocks, Cortés (2000) identified most sharks to have k ≤ 0.2. Also, Frisk et al. (2001) found that large elasmobranchs (TL > 200 cm, excluding requiem sharks) have k averaging to 0.11. So, the k value generated by White et al. (2014) seems to be too high. Meanwhile, our results (k = 0.095) seem to be more realistic. There are some issues with White et al. (2014) results. Firstly, their data only cover a small range of total length, and the maximum length observed is too small. Secondly, the data is of small sample size as also admitted by White et al. (2014) themselves.
For R. ancylostoma, the estimated \({{L}_{\infty }}\) is 283.5 cm. However, no definite growth model can be established due to the inability to generate growth rate parameter (k). To anticipate this, multiple k values (0.08, 0.12, 0.16 and 0.20) have been used. The range of 0.08−0.20 was resulted from accounting for the metadata of Cortés (2000) and the average of k by Frisk et al. (2001). Also, Frisk et al. (2001) and Cortés (2000) argue that larger elasmobranch tends to have lower k. Asymptotic length (\({{L}_{\infty }}\)) of R. australiae appears to be higher than R. ancylostoma. So, expecting the k value for R. anclylostoma to be higher than 0.095 is reasonable, again, justifying the k range we made. In addition, this range of k is appropriate in terms of the plausibility of amax and M estimates derived from k. If k is set too low (<0.08), the resulting amax becomes too high, which is unrealistic. Similarly, if k is too high (>0.20), the resulting M becomes unreasonably too high (see subsection “Survivorship”). The growth curve for R. australiae along with age−length pairs from White et al. (2014) is presented in Fig. 4a, while the growth curve for R. ancylostoma for various k values is displayed in Fig. 4b.
The \({{L}_{\infty }}\) estimate for R. australiae is a bit higher than that of R. ancylostoma. This is because the maximum observed TL for R. australiae is also higher (323 vs 297 cm) and the estimations of \({{L}_{\infty }}\) in this study was entirely based on maximum length.
Survivorship
Six methods for estimating natural mortality rates (M) generated survivorship values in scenario without fishing, as displayed in Tables 3, 4. Estimates of survival rates for R. australiae are broadly consistent with each other (Table 3). The differences across different methods were small, the survivorship ranges between 0.804−0.901, indicating the robustness of the estimates. These values told us that each year, as many as 9.9−19.6% of individuals in a cohort die of natural causes. In scenario with fishing, survivorship is still high (0.715), indicating that the fishing mortality rate (F) is still low. Coupled with survivorship in the unfished population, in the fished population of R. australiae, only as many as 8.9−18.6% of individuals die of fishing after the cohort is fully selected by fishing gears. So, if at particular time a fully selected cohort consist of 1000 individuals, then one year later the cohort will leave 715 individuals surviving, with 99−196 individuals die of natural causes and 89–186 individuals of fishing. Of course, it is just a crude estimate, particularly because constant M and Z across different ages and years have been assumed which should be given cautions given fluctuations both in ecosystem dynamics and fishing intensities over time Secondly, the example above takes one fixed Z, when, in fact, the estimate is random with positive variance.
Noticeable is that difference in k leads to different survivorship estimate for R. ancylostoma in unfished and fished populations (Table 4). All methods for estimating natural mortality rates (accordingly survival rates in unfished population) depend on k. They are the functions of k either directly or indirectly. Pauly’s method and one of Then et al.’s method are functions in k, while the rest are functions in amax, which in turn depend on k as well (due to the absence of empirical amax). There is a trend of monotonically increasing natural mortality rate (M) as k increases, accordingly survivorships in unfished populations decrease. The case for the fished population is no different. Total mortality rate (Z) was estimated from a length−based linearized catch curve involving inverse von Bertalanffy function, which definitely uses k in the calculation. The trend is similar i.e as k increases, Z increases, so survivorship in fished populations decreases.
However, it can be seen for the same k value, the estimates of survivorship for R. ancylostoma cohort in scenarios without fishing agree among different methods, similar to the case for R. australiae. As k gets bigger, the survivorship estimate gets lower. In the fished population the decrease in survivorship is more apparent. For k = 0.2, the survivorship is only 0.456, which means each year less than half of the cohort survives. The survival rate of R. ancylostoma is lower than that of R. australiae in most k values, except for k = 0.08. The difference gets bigger for higher k. This holds for both unfished and fished scenarios.
The survival rates for fished populations in the two species were based on catch-curve analysis, which assumes fish recruitments as relatively constant over time (Hilborn and Walters, 1992; Sparre and Venema, 1998; Hisano et al., 2011). This assumption is unlikely because low fecundities in elasmobranch (moreover in these two wedgefishes) makes the stock-recruitment relationship more direct (Hoenig and Gruber, 1990; Smith et al., 1998; Cortés, 2011). So, the number of offsprings should be highly dependent on the number of mothers. However, currently, linearized catch-curve is the best method for estimating total mortality rates given the limitation in data. Similar to this work, Hisano et al. (2011) also used catch-curve analysis to derive mortality rates for their demographic model in the absence of complete data.
Demographic Analysis
The results of the matrix model for R. australiae (Table 5) show λ > 1 (95% CI lies to the right of 1) or r > 0 (95% CI lies the right of 0) for both both with- and without-fishing scenarios, indicating that populations positively grow. Based on λ values, the unfished population increased at a rate of 30% per year (1 year RC) or 23% per year (2 years RC), revealing the relatively high productivity of this species. Despite using different k values, D’Alberto et al. (2019) also found the equally high productivity for unfished population of R. australiae, with instantaneous growth rate (r) between 0.22−0.49, consistent with this present study. This work also shows fished population increases at a rate of 11 or 5% per year for 1 or 2 years RC, respectively.
Based on R0 values, an individual female in the unfished population on average produces 16.5 offsprings (1 year RC) or 8.92 offsprings (2 years RC) during its lifetime. Meanwhile, a female in the fished population produces only 2.78 or 1.62 offsprings during its lifetime (with 1 or 2 years RC). In all scenarios, R0 > 1, showing that each single mother is being replaced by more than 1 offspring. This explains why the population grows both in with- and without-fishing scenarios.
Ignoring the density-dependence mechanism, the population will double every certain period. Unfished population will double every 2.6 years (1 year RC) or 3.4 years (2 years RC), which is very fast. Meanwhile, the fished population takes 6.5 or 13.4 years to double in 1 or 2 years RC, which is still fast.
Positive growth in the fished population indicates that current exploitation has not endangered the stock of R. australiae in western Indonesian inner waters. In addition, the population of R. australiae increases quickly, indicating this species as productive. In all scenarios, the doubling time is less than 20 years (Table 5) which is quite short. Ten years projections as displayed in Fig. 5 confirm this high increase of R. australiae’s population. Within ten years, the populations will have grown to a very high level. In without-fishing scenarios the population will grow to nearly 1400% of initial abundance for 1 year RC and nearly 800% for 2 years RC which are exceptionally high. In fished scenarios, the population will grow to nearly 300 and 170% for 1 and 2 years RC respectively. Within 10 years the populations’ increase is more than 100% in almost all scenarios, except the fished population with 2 years RC, 70%. This high increase will work only for a low initial population. For population size near carrying capacity, the density dependent mechanism due to limited food and space will hinder this high long-term growth rate. However, how big the actual long-term population is, is not very important. This is just to show that R. australiae has high productivity, hence, helps explain why it can withstand the current level of exploitation.
The analysis of matrix models for R. ancylostoma (Table 6) shows only population without fishing is positively growing, indicated from λ > 1 (95% CI lies to the right of 1 except for k = 0.20 with two years RC) and the corresponding r values. This unfished population only increases slightly, in contrast to R. australiae, which grows significantly. The annual increases vary according to k values, ranging 11–15% per year with 1 year RC or 4–9% per year with 2 years RC. While natural mortality rates increases, λ or r does not show a clear trend by the increase of k.
On the other hand, the fished populations reveal decline in most cases as represented by λ < 1 or r < 0. The exception is the case k = 0.08 with 1 year RC showing almost stagnant population. However, even for this case, the wide 95% CI of λ or r indicate the probability of population declining is high. For other k values, the decrease of the fish population is as many as 1–21 or 4–26% per year with 1 and 2 years RC respectively. There seem to be decreasing trends of λ or r with the increasing k. This is likely to be related to significantly increased total mortality rate Z.
According to Table 4, it is clear that the decrease in the survival rate of the fished population (driven by the increase in Z) is more significant. Bradshaw et al. (2018) reported that survival rate has a big effect on population growth in copper shark (Carcharhinus brachyurus). Meanwhile, while in unfished populations survival rate also decreases, the decrease is not significant. Other vital rates might have compensated the effect of increasing M in unfished populations of R. ancylostoma. Age at maturity (am) is l ikely to be the compensator.
According to Cortés (2002) and Pardo et al. (2018), age at maturity is negatively correlated with population growth. This is particularly sensible because in this study am are derived from Lm making use of k in the processes. As k increases, this quantity decreases (Table 2), making R. ancylostoma reproduce earlier. Early reproduction schedule provides significant additions to offspring because the abundance of younger mothers is significantly higher than older ones due to progressive mortality to a cohort. Even though at the same time longevity (amax) decreases as well which consequently reduces the reproductive lifespan, variation in longevity has only little effect on population growth (Smith et al., 1998; Hisano et al., 2011). This is because older mature fish have been significantly reduced. So they do not produce significantly more offspring.
For each scenario of unfished and fished population with two different RC, the precision of λ (or r) estimate as represented by the 95% CI tend to be negatively correlated with k values. This seems to be related to change in age at maturity (am). Cortés (2002) also found that precision of λ estimates across various shark species decreases with decreasing am, which agree with this study on R. ancylostoma.
The net reproductive output (R0) of R. ancylostoma in the unfished population also varies by k values, ranging from 2.35 to 6.27 offsprings (1 year RC) or 1.36 –3.35 offsprings (2 years RC) per female. While the corresponding fished population produces 0.16–1.03 offsprings or 0.10–0.58 offsprings per female with 1 or 2 years RC respectively, which is very small. R0 ≤ 1 in the with-fishing scenario means that every single mother in the population is replaced by less than one offspring, explaining why the population in this scenario decays. Different from λ, in both unfished and fished populations R0 shows a clear pattern of decrease with the increase of k. This should be clear from its formula. R0 is the function of only survival rate (Sa) and fecundity (fa). While fecundity is constant across all k, survival rates (both in with- and without-fishing scenarios) decreases as k increases.
Due to the low rate of population increase, the unfished population of R. ancylostoma takes longer time to double. Across all k values, the doubling time is between 5−7 years with 1 year RC or 8–19 years with 2 years RC, significantly longer than their counterparts in R. australiae. Since t×2 is derived from r values in unfished populations, then similar with r, it does not show a trend as k increases. Meanwhile, for the with-fishing scenario, the population is undergoing a decline. With the current exploitation level (and ignoring the compensation mechanism), the population will be collapsed after a certain period. Similar to t×2, this collapse time (tcoll) varies according to k values, i.e 19–488 years with 1 year RC or 15–125 years with 2 years RC. Furthermore, tcoll is also derived from r values in fished populations. So, tcoll shows a trend of decrease as k increases.
R. ancylostoma’s population only shows an increase when fishing does not occur. However, this increase is significantly lower than that of R. australiae. Ten years projections (Fig. 6) shows that within 10 years the population of R. ancylostoma grows to less than 400% of initial population in 1 year RC and no more than 250% in 2 years RC, depending on k value. These are much lower than R. australiae, more than 1300 and 700% (Fig. 5). Similar to λ, the projected population size in the next 10 years fluctuates but does not show any trend with the increase of k. This is sensible because projections were entirely based on λ.
Ten years projection of female population of Rhina ancylostoma with the associated 95% confidence interval (CI) for scenarios (1) k = 0.08, (2) k = 0.12, (3) k = 0.16, (4) k = 0.20 and (a) unfished population with 1 year reproductive cycle (RC), (b) fished population with 1 year RC, (c) unfished population with 2 years RC, (d) fished population with 2 years RC.
Theoretically, unfished population should always increase or at least stationary (λ ≥ 1, r ≥ 0) in order for the population to ever exist. Otherwise, the population will have been collapsed a long time ago. However, using a similar matrix model, Cortés (2002) really found λ < 1 in unfished populations for some species of Carcharhinus, Squalus, and Alopias. Particular care should be taken toward this. Cortés (2002) suggested that this unrealistic growth rate is partly due to violations in assumptions of demographic analysis. Biased life-history parameters used may also lead to this negative growth of unfished populations.
When current level of fishing is introduced, the population of R. ancylostoma generally declines. Ten years projection of female population (Fig. 6) confirms this. In almost all scenarios of fished population, the trajectories are going downward, showing the population gets lower and lower over time. Similar to λ, the projected population decreases more steeply as k increases. For k = 0.2, the population drops to very low levels, less than 10% for 1 year RC and less than 5% for 2 years RC. This corroborates the result in Table 6 that if k = 0.20 holds, the collapse of R. ancylostoma may occur within 20 years. This result is alarming. Even though this collapse is not mathematically equivalent to extinction due to the definition of tcoll (as time by which the population is depleted to less than 1% of initial abundance), but when this very low level of population is achieved, the risk for local extinction is high ecologically due to factors such as environmental stochasticity or Allee effect (Berec et al., 2007; Holden and McDonald-Madden, 2017).
Several things are suspected of leading to a difference in the productivity of R. australiae and R. ancylostoma. The first possible factor is the difference in survival rates. Tables 1 and 4 show that R. australiae tend to have a higher survival rate than R. ancylostoma both in unfished and fished populations. This has been exemplified by R. ancylostoma (under various k values), in which higher survival rate leads to higher population growth, which was also supported by Bradshaw et al. (2018). Second and especially noticeable is the difference in fecundity in the two species. R. australiae is significantly more fecund than the latter species. It is easily understandable as fecundity is a very important factor influencing the number of offsprings produced, which ultimately determines how fast the population grows. Tsai et al. (2014) also found that survival rate and fecundity contribute significantly to population growth in shortfin mako shark (Isurus oxyrinchus). So, it is likely to be the case that higher fecundity and relatively higher survival rate leads to higher population growth in R. australiae compared to R. ancylostoma. This significant influence of fecundity was also confirmed by Pardo et al. (2018) and Cortés (2002). However, other than fecundity, Pardo et al. (2018) and Cortés (2002) also found age at maturity to be another important factor determining population growth and in Cortés, even more dominant than a fecundity. Generally, age at maturity is negatively correlated with the population growth rate. Unfortunately, the comparison of age at maturity in these two species is hindered by its uncertainty in R. ancylostoma resulted from uncertainty in growth parameter k. On the other hand, R. ancylostoma clearly has larger size at maturity than R. australiae (180 vs. 155 cm TL). This might be another factor leading to a lower population increase in R. ancylostoma. According to Smith et al. (1998), the instantaneous rate of population increase is closely related to age and size at maturity.
Following the categorization by Frisk et al. (2001), both R. australiae and R. ancylostoma belong to large elasmobranch (>2 m TL). It was hypothesized that large elasmobranch tend to have low productivity, making them more prone to population depletion (Smith et al., 1998; Fisk et al., 2001; Dulvy and Reynolds, 2002). The case of R. ancylostoma seems to follow this general theory. In contrast, Cortés (2002) found no correlation between body size and population growth rate based on 38 shark species. In addition, Garcia et al. (2008) found only a weak influence of body size to extinction risk and suggested this is due to a correlation between body size and other more meaningful life-history traits. The case of R. australiae seems to favor these two studies rather than the above general theory.
The result of matrix models infers that for R. australiae, the current level of fishing can be maintained to give maximum benefit for the fishermen. While for R. ancylostoma, a substantial reduction in catch/fishing effort are needed in order for the population to persist in the long run. Otherwise, exploitation is driving the population of R. ancylostoma to severe depletion, and several years later, local extinction may happen.
The status of R. australiae which this study inferred seems to contradict its status as Critically Endangered in IUCN Red List. For this, several things should be noted. Firstly, the IUCN Red List status measures the global status of R. australiae given the global fishing pressure. Individual stocks can have different conditions. Our study and that of D’Alberto et al. (2019) agree that naturally R. australiae is productive in term of unfished population growth. Fish with high productivity can still be endangered if massive exploitation is imposed. However, closer look to R. australiae stock in western Indonesian inner waters, shows that exploitation is not so high that it can still withstand the current exploitation level.
Secondly, in IUCN Red List assessment, Kyne et al. (2019) conclude the status of R. australiae as Critically Endangered based on the global population decline they claimed to be more than 80% during 3 generations. However, this magnitude of population decline is crude. This does not come from formal stock assessment, rather from historical catch records from several places, with the assumption that catch reflects population abundance. This assumption is controversial (e.g Pauly et al., 2013) which Kyne et al. (2019) are also aware of. As an example, the declining trend of wedgefish catch from Indonesia as shown in Fig. 2, if it really happened, is due to the decrease in the number of vessels operating (Simeon et al., 2019), instead of decline in abundance. For comparison, analyses on the data from southern Indonesian waters (eastern Indian Ocean) show that the catch per unit effort (CPUE) of R. australiae has been increasing during 2014−2020, not decreasing. Furthermore, even if we insist to use catch as the proxy for abundance, almost nowhere catch report for wedgefish are segregated per species. What Kyne et al. (2019) observed is, at best, the declining catch of aggregated wedgefish, instead of R. australiae alone. Worsely, in Southeast Asia, the signal of decline in wedgefish catch usually comes from the observed declines of overall demersal fisheries (Stobutzki et al., 2006). With this, it is difficult to see the real picture of catch trajectory for R. australiae alone. It is probable that while the catch of other demersal fish are declining, the one for R. australiae is not or at least the decline is not as significant as other fish. The likelihood increases when it is revealed that different species of wedgefish have very different productivities. Also, Kyne et al. (2019) used simplistic formula to compute the generation length (GL, as explained in Kyne et al., 2020) and got GL 15 years for R. australiae. Using this value, they estimated the global decline during 3 generations to be >80%. Using a more formal formula borrowed from life table analysis (Simpfendorfer, 2005), we computed the GL to be 8−12 years. We do not have access to Kyne et al. (2019) data to be able to recalculate the global decline of R. australiae. However, with this updated GL, we believe the decline will be less than 80% even if historical catch data of Kyne et al. (2019) is used without cautions.
Elasticity Analysis
In order to better understand the influence of demographic uncertainties on population growth (λ or r), Tables 7, 8 and Figs. 7, 8 summarize the results of elasticity analysis. Based on elasticity per age, younger individuals of both R. australiae and R. ancylostoma have higher elasticities regardless of scenarios of population with or without fishing (Figs. 7, 8). This holds in R. ancylostoma in all k scenarios. In both species, the peaks occur at age 0 up to (including) age at first pupping ap. After that, the elasticities start to decline. The older the fish, the lower the elasticities of each age to population growth.
Disentangling elasticities of fecundity and survival, it is revealed that the survival of juvenile (age 0 up to age before pupping) has the highest elasticity in both wedgefish (Tables 7, 8). The elasticity of juvenile’s survival is significantly higher than those of adult’s survival and fecundity. This result agrees with the finding of Cortés (2002) on various shark species and Heppel et al. (1999) on leopard shark (Triakis semifasciata) and angel shark (Squatina californica). In all cases, elasticity of survival of age at first pupping ap has started to decline; ap being still at the peak of elasticity (Figs. 7, 8) is resulted from the sum of elasticities of survival and fecundity at that age.
Population growth rates in both with- and without-fishing scenarios are very sensitive to the survival of young fish (age 0 up to age before pupping). Small variations in survival rates of these ages can alter population growth rates dramatically. Since the survival of R. ancylostoma is generally lower than R. australiae, the same pattern certainly occurs for juveniles. So, juveniles (as well as adults) of R. ancylostoma tend to have lower survival rates compared to R. australiae. This justifies the previous claim that the lower population growth of R. ancylostoma (even negative growth in fished population) is partly caused by its lower survival rate compared to R. australiae.
We also suspected that the lower R. ancylostoma’s fecundity become another factor leading to its lower population growth. Despite being not as significant as survival rate, positive elasticities of fecundities (Tables 7, 8) indicates that fecundity really has an influence on the population growth.
That elasticity of juvenile fish is high is sensible considering the fact that abundant juveniles determine the availability of mature individuals. If young individuals are depleted, then only a few fish (if any) reach the reproduction stage, while reproduction is ultimately the reason why the population even exists. It’s likely to be the reason why age at first pupping is still at the peak of elasticity.
However, while older fish are assumed to reproduce continuously, Figs. 7 and 8 clearly show they have a decreasingly lower effect on population growth both in terms of survival or fecundity. First, enough offsprings may have been produced by younger mothers. So, more offspring from older mothers are not really needed to sustain the population. Second, while it is true that older mothers continuously give birth, their abundance has been reduced significantly. So, not many offspring can be produced by older mothers. This is especially true when fecundity per capita stays constant over ages (which is the case for this two wedgefishes). This is different from the finding of Tsai et al. (2010) in which the highest elasticity in pelagic thresher shark (Alopias pelagicus) occurs at the adult stage instead of younger fish. However, they used the stage-based matrix, with only four stages (neonate, juvenile, subadult, and adult) assumed to represent up to 30 age classes. Mollet and Cailliet (2002) reminded to caution elasticities from stage-based models as they usually underestimate the elasticity of juvenile survival and overestimate the one of the adults. Using the age-based matrix, Cortés (2002) found that the case where adult stages have higher elasticity occurs for sharks with early age at maturity.
According to this study, the existence of juveniles and young mothers seems determinant to maintain the population level. In this regard, protection toward young fish (juvenile up to the age at first reproduction) will be the most suitable strategy. For R. australiae, protection up to age seven years old is recommended. However, for R. ancylostoma, uncertainty in age at maturity generated from uncertainty in somatic growth parameter (k) complicates the management recommendation. Being precautionary, protection up to age 13 for Rhina ancylostoma may be implemented with the risk of sacrificing potential benefits from fisheries. These protections towards younger fish can be implemented by limiting or even prohibiting fishing activities in nursery areas.
Further Studies
Some aspects of the present work were based on simplistic assumptions and incomplete information on vital life-history parameters. Therefore, the result of this study should be regarded as preliminary, and further studies should be conducted both to provide more complete information on vital parameters and to improve stock assessments using this better information.
Throughout this study, uniform distributions have been used to represent uncertainties on some vital rates: age at first pupping, fecundity, and longevity driven by lack of information. A uniform distribution that has no mode is unlikely and contains greater uncertainties that are propagated to uncertainty in population growth rate (λ and r). The lack of information on sex ratio and RC also forces to make simplistic assumptions, which may lead to inaccurate growth estimates. Finding empirical values and distribution of these vital parameters can lead to better and more accurate population growth estimates.
Age and growth studies for both R. australiae and R. ancylostoma are also still needed to generate more reliable growth parameters (\({{L}_{\infty }}\) and k). In R. australiae, current growth parameters were based on incomplete data of White et al. (2014). The importance is twofold for R. ancylostoma in which age and growth study is not available at all.
For mortality, natural mortality rates seem to be the most difficult to estimate. However, this should not bother much because the population growth derived from demographic model is usually less sensitive to natural mortality (Pardo et al., 2018). Instead, further study should focus on fishing mortality. Once a better catch recording system (by management authority) is achieved, more thorough mortality estimation methods such as virtual population analysis (VPA) should be attempted in place of the current linearized catch curve, which is less realistic.
In order to protect juveniles of both R. australiae and R. ancylostoma as recommended in this work, a study on nursery grounds for both species is important. Last but not least, throughout this study, R. australiae and R. ancylostoma in western Indonesian inner waters have been assumed to form unit stocks. To what extent this assumption holds remains uncertain. Tagging or genetic study can help find out the stock structure of both fish in these areas.
CONCLUSIONS
This study concludes that R. australiae is productive, while R. ancylostoma is not sufficiently productive in terms of population growth. The current level of fishing has not endangered the population of R. australiae in western Indonesian inner waters, so it can be maintained to give maximum benefit to fishermen and fisheries communities. Differently, the population of R. ancylostoma cannot withstand current levels of exploitation, so the substantial reduction in fishing is required to preserve its population in this region. Furthermore, protecting young fish (juveniles up to the age at first reproduction) is recommended for both wedgefish species in order for populations to sustain. For R. australiae, fish of age less than equal seven years should be protected, while for R. ancylostoma age-based restriction cannot be set yet due to lack of information on age at first reproduction.
REFERENCES
Alverson, D.L. and Carney, M.J., A graphic review of the growth and decay of population cohorts, ICES J. Mar. Sci., 1975, vol 36, no. 2, pp. 133–143. https://doi.org/10.1093/icesjms/36.2.133
Berec, L., Angulo, E., and Courchamp, F., Multiple Allee effects and population management, Trends Ecol. Evol., 2007, vol. 22, no. 4, pp. 185–191. https://doi.org/10.1016/j.tree.2006.12.002
Blaber, S.J.M., Dichmont, C.M., White, W., Buckworth, R., Sadiyah, L., Iskandar, B., Nurhakim, S., Pillans, R., Andamari, R., Dharmadi, and Fahmi, Elasmobranchs in southern Indonesian fisheries: the fisheries, the status of the stocks and management options, Rev. Fish. Biol. Fish., 2009, vol. 19, pp. 367–391. https://doi.org/10.1007/s11160-009-9110-9
Borrell, A., Cardona, L., Kumarran, R.P., and Aguilar, A., Trophic ecology of elasmobranchs caught off Gujarat, India, as inferred from stable isotopes, ICES J. Mar. Sci., 2011, vol. 68, no. 3, pp. 547–554. https://doi.org/10.1093/icesjms/fsq170
Braccini, M., Molony, B., and Blay, N., Patterns in abundance and size of sharks in northwestern Australia: cause for optimism, ICES J. Mar. Sci., 2020, vol. 77, no. 1, pp. 72–82. https://doi.org/10.1093/icesjms/fsz187
Bradshaw, C.J.A., Prowse, T.A.A., Drew, M., Gillanders, B.M., Donnellan, S.C., and Huveneers, C, Predicting sustainable shark harvests when stock assessments are lacking, ICES J. Mar. Sci., 2018, vol. 75, no. 5, pp. 1591–1601. https://doi.org/10.1093/icesjms/fsy031
Campana, S.E., Ferretti, F., and Rosenberg, A., Sharks and other elasmobranchs, in The First Global Integrated Marine Assessment: World Ocean Assessment I, Inniss, L., et al., Eds., Cambridge: Cambridge Univ. Press, 2017, pp. 781–788. https://doi.org/10.1017/9781108186148.050
Cortés, E., Life history patterns and correlations in sharks, Rev. Fish. Sci., 2000, vol. 8, no. 4, pp. 299–344. https://doi.org/10.1080/10408340308951115
Cortés, E., Incorporating uncertainty into demographic modeling: application to shark populations and their conservation, Conserv. Biol., 2002, vol. 16, pp. 1048–1062. https://doi.org/10.1046/j.1523-1739.2002.00423.x
Cortés, E., An overview of approaches used to assess the status of shark populations: experiences from the US and ICCAT in the Atlantic Ocean, in The 7th Session of The Working Party on Ecosystems and Bycatch, Victoria: Indian Ocean Tuna Comm., 2011.
D’Alberto, B.M., Carlson, J.K., Pardo, S.A., and Simpfendorfer, C.A., Population productivity of shovelnose rays: inferring the potential for recovery, PLoS One, 2019, vol. 14, no. 11, art. ID e0225183. https://doi.org/10.1371/journal.pone.0225183
Devadoss, P. and Batcha, H., Some observations on the rare bow-mouth guitarfish Rhina ancylostoma, Mar. Fish. Inf. Serv., 1995, vol. 138, pp. 10–11.
Dharmadi, Fahmi, and White, W.T., Biodiversity of sharks and rays in south eastern Indonesia, Indones. Fish. Res. J., 2009, vol. 15, no. 2, pp. 17–28. https://doi.org/10.15578/ifrj.15.2.2009.17-28
Dulvy, N.K., Fowler, S.L., Musick, J.A., Cavanagh, R.D., Kyne, P.M., Harrison, L.R., Carlson, J.K., Davidson, L.N.K., Fordham, S.V., Francis, M.P., Pollock, C.M., Simpfendorfer, C.A., Burgess, G.H., Carpenter, K.E., Compagno, L.J.V., et al., Extinction risk and conservation of the world’s sharks and rays, eLife, 2014, vol. 3, art. ID e00590.
Dulvy, N.K. and Reynolds, J.D., Predicting extinction vulnerability in skates, Conserv. Biol., 2002, vol. 16, pp. 440–450. https://doi.org/10.1046/j.1523-1739.2002.00416.x
Elzhov, T.V., Mullen, K.M., Spiess, A.-N., and Bolker, B., minpack.lm: R interface to the Levenberg-Marquardt nonlinear least-squares algorithm found in MINPACK, plus support for bounds, R package version 1.2-1, 2016. https://CRAN.R-project.org/package=minpack.lm.
Fahmi, Dharmadi, Sarmintohadi, Mustika, C., Dermawan, A., Sadili, D., Suharsono, and Kasasiah, A., A Review of the Status of Shark Fisheries and Shark Conservation in Indonesia, Jakarta: Dir. Mar. Aquat. Resour. Conserv., Dir. Gen. Mar., Coasts Small Islands, Ministry Mar. Aff. Fish., 2013.
Frisk, M.G., Miller, T.J., and Fogarty, M.J., Estimation and analysis of biological parameters in elasmobranch fishes: a comparative life history study, Can. J. Fish. Aquat. Sci., 2001, vol. 58, pp. 969–981. https://doi.org/10.1139/f01-051
Froese, R. and Binohlan, C., Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data, J. Fish Biol., 2000, vol. 56, pp. 758–773. https://doi.org/10.1111/j.1095-8649.2000.tb00870.x
Garcia, V.B., Lucifora, L.O., and Myers, R.A., The importance of habitat and life history to extinction risk in sharks, skates, rays and chimaeras, Proc. R. Soc. B, 2008, vol. 275, pp. 83–89. https://doi.org/10.1098/rspb.2007.1295
Gedamke, T., Hoenig, J., Musick, J.A., DuPaul, W.D., and Gruber, S.H., Using demographic models to determine intrinsic rate of increase and sustainable fishing for elasmobranchs: pitfalls, advances and applications, North Am. J. Fish. Manage., 2007, vol. 27, pp. 605–618. https://doi.org/10.1577/M05-157.1
Geng, Z. and Zhu, J., Estimating population growth rate for Indian Ocean blue shark (Prionace glauca) using demographic method, in The 13th Working Party on Ecosystem and Bycatch, San Sebastian: Indian Ocean Tuna Comm., 2017.
Goldman, K.J., Branstetter, S., and Musick, J.A., A re-examination of the age and growth of sand tiger sharks, Carcharhinus taurus, in the western North Atlantic: the importance of ageing protocols and use of multiple back-calculation techniques, Environ. Biol. Fish., 2006, vol. 77, pp. 241–252. https://doi.org/10.1007/s10641-006-9128-y
Heppell, S.S., Crowder, L.B., and Menzel, T.R., Life table analysis of long-lived marine species, with implications for conservation and management, in Life in the Slow Lane: Ecology and Conservation of Long-Lived Marine Animals, Musick, J.A., Ed., Bethesda, MD: Am. Fish. Soc., 1999, pp. 137–148.
Hilborn, R. and Walters, C.J., Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty, New York: Chapman and Hall, 1992.
Hisano, M., Connolly, S.R., and Robbins, W.D., Population growth rates of reef sharks with and without fishing on the Great Barrier Reef: robust estimation with multiple models, PLoS One, 2011, vol. 6, no. 9, art. ID e25028. https://doi.org/10.1371/journal.pone.0025028
Hoenig, J.M., Empirical use of longevity data to estimate mortality rates, Fish. Bull., 1983, vol. 82, pp. 898–903.
Hoenig, J.M. and Gruber, S.H., Life history patterns in elasmobranchs: implications for fisheries management, in Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematic and the Status of the Fisheries, NOAA Tech. Rep. NMFS, no. 90, Pratt, H.L., Jr., et al., Eds., Washington, DC: Natl. Ocean. Atmos. Admin., 1990, pp. 1–16.
Holden, M.H. and McDonald-Madden, E., High prices for rare species can drive large populations extinct: the anthropogenic Allee effect revisited, J. Theor. Biol., 2017, vol. 429, pp. 170–180. https://doi.org/10.1016/j.jtbi.2017.06.019
Jabado, R.W., The fate of the most threatened order of elasmobranchs: shark-like batoids (Rhinopristiformes) in the Arabian Sea and adjacent waters, Fish. Res., 2018, vol. 204, pp. 448–457. https://doi.org/10.1016/j.fishres.2018.03.022
Kadri, H., Marouani, S., Bradai, M.N., Bouain, A., and Morize, E., Age, growth, mortality, longevity and reproductive biology of the white skate, Rostroraja alba (Chondrichthyes: Rajidae) of the Gulf of Gabès (Southern Tunisia, Central Mediterranean), Turk. J. Fish. Aquat. Sci., 2014, vol. 14. pp. 193–204. https://doi.org/10.4194/1303-2712-v14_1_21
Kyne, P.M., Rigby, C.L., Dharmadi, and Jabado, R.W., Rhynchobatus australiae, in The IUCN Red List of Threatened Species, Glanz, 2019, no. e.T41853A68643043. https://doi.org/10.2305/IUCN.UK.20192.RLTS.T4185-3A68643043.en
Kyne, P.M., Jabado, R.W., Rigby, C.L., Dharmadi, Gore, M.A., Pollock, C.M., Herman, K.B., Cheok, J., Ebert, D.A., Simpfendorfer, C.A., and Dulvy, N.K., The thin edge of the wedge: Extremely high extinction risk in wedgefishes and giant guitarfishes, Aquat. Conserv. Mar. Fresh. Ecosyst., 2020, vol. 30, no. 7, pp. 1337–1361. https://doi.org/10.1002/aqc.3331
Kynoch, R.J., Fryer, R.J., and Neat, F.C., A simple technical measure to reduce bycatch and discard of skates and sharks in mixed-species bottom-trawl fisheries, ICES. J. Mar. Sci., 2015, vol. 72, no. 6, pp. 1861–1868. https://doi.org/10.1093/icesjms/fsv037
Last, P.R. and Stevens, J.D., Sharks and Rays of Australia, Canberra: CSIRO, 2009, 2nd ed.
Last, P.R., White, W.T., and Seret, B., Wedgefishes: family Rhinidae, in Rays of the World, Last, P.R., , Eds., Canberra: CSIRO, 2016, pp. 65–76.
Masuda, H., Araga, C., and Yoshino, T., Coastal Fishes of Southern Japan, Tokyo: Tokai Univ. Press, 1975.
Mildenberger, T.K., Taylor, M.H., and Wolff, M., TropFishR: an R package for fisheries analysis with length-frequency data, Methods Ecol. Evol., 2017, vol. 8, no. 11, pp. 1520–1527. https://doi.org/10.1111/2041-210X.12791
Mollet, H.F. and Cailliet, G.M., Comparative population demography of elasmobranchs using life history tables, Leslie matrices and stage-based matrix models, Mar. Freshwater Res., 2002, vol. 53, pp. 503–515. https://doi.org/10.1071/MF01083
Moore, A.B.M., Are guitarfishes the next sawfishes? Extinction risk and an urgent call for conservation action, Endangered Species Res., 2017, vol 34, pp. 75–88. https://doi.org/10.3354/esr00830
Pardo, S.A., Cooper, A.B., Reynolds, J.D., and Dulvy, N.K., Quantifying the known unknowns: estimating maximum intrinsic rate of population increase in the face of uncertainty, ICES J. Mar. Sci., 2018, vol. 75, no. 3, pp. 953–963. https://doi.org/10.1093/icesjms/fsx220
Pauly, D., On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks, ICES J. Mar. Sci., 1980, vol. 39, no. 2, pp. 175–192. https://doi.org/10.1093/icesjms/39.2.175
Pauly, D. and David, N., ELEFAN 1, a BASIC program for the objective extraction of growth parameters from length-frequency data, Meeresforschung, 1981, vol. 28, no. 4, pp. 205–211.
Pauly, D., Hilborn, R. and Branch, T.A., Fisheries: Does catch reflect abundance? Nature, 2013, vol. 494, pp. 303–306. https://doi.org/10.1038/494303a
R Core Team, R: A Language and Environment for Statistical Computing, Vienna: R Found. Stat. Comput., 2019, https://www.R–project.org/.
Raje, S.G., Skate fishery and some biological aspects of five species of skates off Mumbai, Indian J. Fish., 2006, vol. 53, no. 4, pp. 431–439.
Sadri and Yuneni, R.R., Wedgefishes and Guitarfishes Conservation: Fisheries Baseline and Identifying Species Threats through Observer and Enumerator Program: Technical Report, Jakarta: WWF Indonesia, 2019.
Simeon, B.M., Muttaqin, E., Ichsan, M., Tarigan, S., Hernawati, and Yulianto, I., Laporan Teknis: Profil Perikanan Wedgefish di Indonesia, Studi Kasus di Nusa Tenggara Barat dan Aceh, Bogor: WCS–IP, 2019.
Simpfendorfer, C.A., Demographic models: life tables, matrix models and rebound potential, in Management Techniques for Elasmobranch Fisheries, Musick, J.A. and Bonfil, R., Eds., Rome: UN Food Agric. Org., 2005, pp. 143–153.
Smith, S.E., Au, D.W., and Show, C., Intrinsic rebound potential of 26 species of Pacific sharks, Mar. Freshwater Res., 1998, vol. 49, pp. 663–678. https://doi.org/10.1071/MF97135
Sparre, P. and Venema, S.C., Introduction to Tropical Fish Stock Assessment, Part 1: Manual, Rome: UN Food Agric. Org., 1998.
Statistics of Marine Capture Fisheries by Fisheries Management Area (FMA), 2005–2016, Jakarta: Dir. Gen. Capture Fish., Ministry Mar. Aff. Fish., 2017.
Stobutzki, I.C., Miller, M.J., Heales, D.S., and Brewer, D.T., Sustainability of elasmobranchs caught as bycatch in a tropical prawn (shrimp) trawl fishery, Fish. Bull., 2002, vol. 100, pp. 800–821.
Stobutzki, I.C., Silvestre, G.T., Talib, A.A., Krongprom, A., Supongpan, M., Khemakorn, P., Armada, N., and Garces, L.R., Decline of demersal coastal fisheries resources in three developing Asian countries, Fish. Res., 2006, vol. 78, pp. 130–142. https://doi.org/10.1016/j.fishres.2006.02.004
Taylor, C.C., Cod growth and temperature, ICES J. Mar. Sci., 1958, vol. 23, no. 3, pp. 366–70. https://doi.org/10.1093/icesjms/23.3.366
Taylor, M.H. and Mildenberger, T.K., Extending electronic length frequency analysis in R, Fish. Manage. Ecol., 2017, vol. 24, pp. 330–338. https://doi.org/10.1111/fme.12232
Then, A.Y., Hoenig, J.M., Hall, N.G., and Hewitt, D.A., Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species, ICES J. Mar. Sci., 2015, vol. 72, no. 1, pp. 82–92. https://doi.org/10.1093/icesjms/fsu136
Tsai, W.-P., Liu, K.-M., and Joung, S.-J., Demographic analysis of the pelagic thresher shark, Alopias pelagicus, in the north-western Pacific using a stochastic stage-based model, Mar. Freshwater Res., 2010, vol. 61, pp. 1056–1066. https://doi.org/10.1071/MF09303
Tsai, W.-P., Sun, C.-L., Punt, A.E., and Liu, K.-M., Demographic analysis of the shortfin mako shark, Isurus oxyrinchus, in the Northwest Pacific using a two-sex stage-based matrix model, ICES J. Mar. Sci., 2014, vol. 71, no. 7, pp. 1604–1618. https://doi.org/10.1093/icesjms/fsu056
Tull, M., The history of shark fishing in Indonesia, in Historical Perspectives of Fisheries Exploitation in the Indo-Pacific, Christensen, J. and Tull, M., Eds., New York: Springer-Verlag, 2014, pp. 63–81. https://doi.org/10.1007/978-94-017-8727-7_4
Wang, K., Zhang, C., Xu, B., Xue, Y., and Ren, Y., Selecting optimal bin size to account for growth variability in Electronic LEngth Frequency ANalysis (ELEFAN), Fish. Res., 2020, vol. 225, art. ID 105474. https://doi.org/10.1016/j.fishres.2019.105474
Ward-Paige, C.A., A global overview of shark sanctuary regulations and their impact on shark fisheries, Mar. Policy, 2017, vol. 82, pp. 87–97. https://doi.org/10.1016/j.marpol.2017.05.004
Weigmann, S., Contribution to the taxonomy and distribution of eight ray species (Chondrichthyes, Batoidea) from coastal waters of Thailand, Verh. Naturwiss. Ver. Hamburg, 2011, vol. 46, pp. 249–312. https://doi.org/10.5402/2012/860768
White, W.T. and Dharmadi, Species and size compositions and reproductive biology of rays (Chondrichthyes, Batoidea) caught in target and non–target fisheries in eastern Indonesia, J. Fish Biol., 2007, vol. 70, pp. 1809–1837. https://doi.org/10.1111/j.1095-8649.2007.01458.x
White, W.T., Last, P.R., Stevens, J.D., Fahmi, and Dharmadi, Economically Important Sharks and Rays of Indonesia, Canberra: Austr. Centre Int. Agric. Res., 2006.
White, J., Simpfendorfer, C.A., Tobin, A.J., and Heupel, M.R., Age and growth parameters of shark-like batoids, J. Fish Biol., 2014, vol. 84, pp. 1340–1353. https://doi.org/10.1111/jfb.12359
Yuwandana, D.W., Agustina, S., Haqqi, M.B., and Simeon, B.M., Studi awal perikanan pari kekeh (Rhynchobatus sp.) dan pari kikir (Glaucostegus sp.) di Perairan Utara Jawa Tengah, J. Akuatika Indones., 2020, vol. 5, no. 1, pp. 1–6. https://doi.org/10.24198/jaki.v5i1.25938
ACKNOWLEDGMENTS
We thank the enumerators who had been collecting the length data of R. australiae and R. ancylostoma at Tegalsari Coastal Fishing Port (Tegal), Bajomulyo Coastal Fishing Port (Pati), Tasik Agung Coastal Fishing Port (Rembang), Brondong Archipelagic Fishing Port (Lamongan) and Sungai Kakap Fish Landing Port (Pontianak) in the years 2017, 2018 and 2019. A gratitude also goes to Muhammad Fauzan who helped prepare the map in Fig. 1.
Funding
The study was funded by (a) COREMAP-CTI through Priority Research Program of Research Center for Oceanography, Indonesian Institute of Sciences, Budgetary Year 2018 and 2019, (b) DIPA of Regional Office for Marine and Coastal Resources Management Denpasar, Ministry of Marine Affairs and Fisheries, Budgetary Year 2017−2019, and (c) Shark Conservation Fund (SCF) through Wildlife Conservation Society (WCS) Indonesia Program and REKAM Nusantara Foundation, Budgetary Year 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Kurniawan, W., Oktaviyani, S., Simeon, B.M. et al. Population Status of Two Wedgefish Species in Western Indonesian Inner Waters, Inferred from Demographic Models with Limited Data. J. Ichthyol. 61, 433–451 (2021). https://doi.org/10.1134/S003294522103005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003294522103005X