Abstract
In the present study, for the first time we isolated and characterized the complete mitochondrial genome sequence of the endangered Farsi killifish Aphanius farsicus by long polymerase chain reaction amplification, and primer walking methods. The circular mitogenome of A. farsicus consisting of 16 485 base pairs encodes 13 polypeptides (protein-coding genes), the 12S and 16S ribosomal RNAs, and 22 transfer RNAs and an 884 bp D-loop control region. These genes are ordered in the same way as most other vertebrates. The overall nucleotide composition of this genome was 27.09 for A; 27.87 for T; 16.89 for G; and 28.14% for C (GC content of 45%, and AT content of 55%). The genus Aphanius has already been in the family Cyprinodontidae. However, the name Aphaniidae has recently been proposed as valid family for the members of the genus Aphanius (the Western Palaearctic killifishes), while the family Cyprinodontidae is restricted to the New World genera such as Cyprinodon, Floridichthys and Jordanella. Based on the phylogenetic relationships achieved in the present study, we recommend that the validation of family Aphaniidae still needs more phylogenetic supports, and this can be investigated by adding more sequences of the Aphanius species. The availability of this mitogenome will also provide a set of useful data for studying on population genetic diversity and molecular evolution and facilitate evaluations of A. farsicus genetic structure for management and conservation of this endangered species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The complete mitochondrial DNA (mtDNA) sequence of vertebrates is a circular molecule with a length of about 16–19 kb (Anderson et al., 1981; Boore, 1999). The mitochondrial genome is frequently used for phylogenetic studies and population genetic analyses because of its compressed gene organization, fast evolutionary rate, maternal inheritance and lack of genetic recombination compared with the nuclear genome (Avise, 1994; Miya et al., 2001).
In recent years, complete mitochondrial DNA sequences have been extensively used to reconstruct the phylogeny of higher-level taxa (e.g. Kim et al., 2004; Jondeung and Karinthanyakit, 2015; Quezada-Romegialli et al., 2015).
The genus Aphanius has already been placed in the family Cyprinodontidae. However, based on the outcomes of recent molecular studies (e.g., Pohl et al., 2015; Helmstetter et al., 2016; Reznick et al., 2017), Aphanius is more closely related to Valencia, the livebearers of the families Anablepidae and Poeciliidae, and the African lampeyes (Aplocheilichthys and related genera), than to other genera in Cyprinodontidae. Therefore, Freyhof et al. (2017) concluded that the family Cyprinodontidae, as proposed by Parenti (1981), is polyphyletic, and proposed Aphaniidae as a valid family name for genus Aphanius (the Western Palaearctic killifishes).
The members of genus Aphanius are widely distributed throughout the Old World (Western Palaearctic and Near East) (Nelson et al., 2016; Freyhof et al., 2017). These fishes are commonly known as killifish or tooth-carps (Wildekamp, 1993). Among vertebrates, the family Aphaniidae represents a group that is particularly useful to study the micro-evolutionary processes shaping patterns of genetic structure and of geographic variation in natural populations, or of speciation processes associated to unstable ecological conditions (Villwock, 1976; Ferrito et al., 2007).
The different Aphanius species inhabit wide range of coastal and landlocked habitats in the drainages of Mediterranean, Red Sea and the Persian Gulf basins (Widemakp, 1993). In its native distribution ranges, 34 species have been recorded for the genus Aphanius so far (Wildekamp, 1993; Teimori et al., 2016; Van der Laan and Fricke, 2018).
By considering its diversity and biogeographic distribution, central Anatolia and Iran are known to show the highest diversity of Aphanius members (Hrbek and Meyer, 2003; Teimori, 2013; Freyhof et al., 2017; Teimori et al., 2018). Currently, 15 extant and one fossil Aphanius species have been discovered and reported from Iran (e.g. Coad, 2000, 2009; Hrbek et al., 2006; Gholami et al., 2014; Esmaeili et al., 2014; Teimori et al., 2012, 2014, 2018) in which 12 species are endemic to this country (Teimori et al., 2016). Therefore, the notable feature of Iranian killifish diversity is its high endemicity. Most of the endemic Aphanius species in this country are distributed in the closed endorheic basins associated with the mountainous regions of Zagros, and characterized by small population size (Gholami et al., 2014; Teimori et al., 2016).
Aphanius farsicus (Fig. 1) is an endemic killifish in the endorheic Lake Maharlu Basin, Southern Iran (Teimori et al., 2011; Esmaeili et al., 2016). It is found in freshwater streams and springs and in the springs of varying saline content or saline influence from hypersaline chloride Lake Maharlu (Esmaeili et al., 2016).
Several studies have used mtDNA to study genetic diversity and phylogeny and evolution of Aphanius species so far. Yaripour et al. (2017) by applying five microsatellite markers studied genetic structure of Aphanius farsicus from the Maharlu Lake basin in Southern Iran. Another study by Salimi et al (2018) investigated genetic diversity in two Aphanius species (A. ginaonis and A. stoliczkanus) in southern Iran by using PCR-RFLP technique. Angeletti et al. (2010) studied genetic diversity of Aphanius fasciatus and found that the genetic variability of this species strongly reduced through time. Also, several previous studies (e.g. Hrbek and Meyer, 2003; Teimori et al., 2012, 2013, 2014, 2018; Esmaeili et al., 2014) investigated phylogenetic relationships, and evolutionary history of different Aphanius species and populations. Therefore, these studies show the value of mtDNA markers for population genetic structure, stock identification, phylogeny, evolution and conservation genetics.
For that reason, in this study, for the first time we sequenced, and characterized the complete mtDNA genome of the endangered killifish A. farsicus. Additionally, we conducted phylogenetic analyses based on the complete mitochondrial genome with the purpose of investigating the phylogenetic position of A. farsicus within the order Cyprinodontiformes.
MATERIALS AND METHODS
Sample Collection and DNA Extraction
The sample of Aphanius farsicus was collected by hand net from a small spring around the Lake Maharlu in Southern Iran. The species were identified at the field by observing morphological characters. A piece of muscle tissue was removed from the individual and preserved in 95% ethanol for DNA extraction. Total genomic DNA from muscle tissue was extracted using standard protocol of FavorPrep Tissue Genomic DNA ExtractionMini Kit (FAVORGEN).
Primers Design and Sequencing
The complete mitogenome of A. farsicus was amplified using a long polymerase chain reaction amplification (long PCR-technique) (Miya and Nishida, 1999; Miya and Nishida, 2000). Six sets of primers (Table 1) were designed based on multiple alignments of the conserved region of the complete mitochondrial DNA sequences of other Cyprinodontiformes extracted from NCBI; including Orestias ascotanensis (NC_027582) (Quezada-Romegialli et al., 2016), Cyprinodon variegatus (KR061357) (Barcelon and Lema, 2016) and Jordanella floridae (AP006778) (Setiamarga et al., 2008) as well as the partial sequence of 12S ribosomal RNA gene, the complete sequence of tRNA-Val gene, and the partial sequence of 16S ribosomal RNA gene which were previously determined for Aphanius persicus (AY593493) (Hrbek et al., 2006) (now A. farsicus, Teimori et al., 2011).
Thereafter, 25 normal PCR primer sets were designed to obtain contiguous, overlapping segments of the entire mitogenome (Table 2).
The NCBI Primer designing tool (https://www. ncbi.nlm.nih.gov/tools/primer-blast/) used to design primers. Primers used in this study were synthesized by DenaZist and Pishgam companies (Tehran-Iran). It was necessary that every two contiguous segments overlapped by at least 50 bp to ascertain the accuracy of sequencing.
Mitochondrial DNA Amplification
Super PCR MasterMix 2X (Yekta Tajhiz Azma-Tehran-Iran) was used to amplify mitochondrial DNA segments using primers listed in Table 1. This reactions were carried out in 25 μl reaction mixture containing 12.5 μL of super PCR MasterMix 2X, 1 μL of DNA template (1 μg), 1 μL of each primer (10 μM), and 9.5 μl sterile distilled H2O. These reactions consisted of an initial denaturing step at 94°C for 4 min, followed by 35 cycles of denaturing at 94°C for 30 s, annealing at 55–58°C (the details of temperature indicated in Table 1) for 45 s and extension at 72°C for 3 min, following with final extension at 72°C for 7 min.
Six mitochondrial DNA segments with ranges from 1.3 to 4 kbp amplified and used as a template in normal PCR using primers listed in Table 2. The Taq DNA Polymerase MasterMix (Ampliqon) used for normal PCR using primer listed in Table 2.
The normal PCR was performed following the standard procedure; containing 12.5 μL of Taq DNA Polymerase MasterMix, 1 μL of amplified mitochondrial segment (achieved by the above mentioned process), 0.5 μL of each primer (10 μM), and add sterile distilled H2O to bring volume to 25 μL. Normal PCR reactions consisted of an initial denaturing step at 94°C for 3 min, following 30 cycles of denaturing at 94°C for 30 s, annealing at 54–58°C (exact temperature indicated in Table 2) for 45 s and extension at 72°C for 1 min, following with final extension at 72°C for 5 min.
Negative controls were included in all PCR amplifications to confirm the absence of contaminants. PCR products were purified sequencing was accomplished by primer walking method (Bioneer-south Korea).
Sequence Editing and Analysis
Sequence trace files were corrected and aligned with the SeaView v. 3.2 (Gouy et al., 2010) and Geneious v. 11.1.5 (Kearse et al., 2012). The locations of 13 protein-coding genes and two rRNA genes were determined by their similarity to published mitogenomes of other killifish species as shown in Table 3, whereas the tRNA genes were identified using the program tRNAscan-SE 2.0 (Lowe and Chan, 2016). Some tRNA genes that could not be found by the tRNAscan-SE, were identified by their secondary structure and their position in the mitogenome (Zhang et al., 2009).
The structure of control region and its conserves motifs were identified by making a comparison with homologous sequences of reported teleost (Quezada-Romegialli et al., 2015; Keepers et al., 2016; Xu et al., 2018).
Phylogenetic Analyses
To clarify the phylogenetic position of A. farsicus within the order Cyprinodontiform, the complete mitochondrial genomes of other 15 killifish species were downloaded from GenBank database (Table 3). In this data set, possible close outgroups in Beloniformes were also choose to root phylogenetic trees (Table 3). We did not include gene ND6 in our data set for phylogenetic analysis owing to its high heterogeneity and poor phylogenetic performance (Miya and Nishida, 2000).
A final data set of 16530 bp including various Cyprinodontiform sequences accompany with two out groups was aligned using Muscle 3.6 (Edgar, 2004) as incorporated in SeaView, under default settings. Finally, phylogenetic position of A. farsicus based on 16 representative killifish complete mitogenome was validated by constructing a maximum likelihood tree with RAxML 7.2.8 (Stamatakis et al., 2008) under a GTR + G + I model, and 1000 rapid bootstrap replicates. The best-fit nucleotide substitution model (GTR + G + I) was selected by AIC (Akaike Information Criterion) using JModelTest (Posada, 2008).
RESULTS AND DISCUSSION
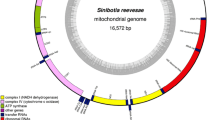
This is the first study that reports complete mitogenome of the genus Aphanius. The complete mitochondrial genome of Aphanius farsicus was 16.530 bp in size, including 13 protein-coding genes, 22 tRNA genes, two rRNA genes, and a putative control region. The gene order of A. farsicus mitochondrial genome was shown in Fig. 2, and the details of organization of the A. farsicus mitogenome are shown in Table 4. There was a non-coding region spanning 884 bp between genes tRNAPhe and tRNAPro with a high A + T content that was identified as a putative control region (Table 4). The complete genome sequence was deposited in GenBank database under the accession number MN578038.
The gene order of mitochondrial DNA of A. farsicus was similar to other killifishes such Orestias ascotanensis (NC_027582), Cyprinodon nevadensisamargosae (KU883631) and Jordanella floridae (AP006778) (Setiamarga et al., 2008; Quezada-Romegialli et al., 2016).
The overall nucleotide composition of mitochondrial genome in A. farsicus was 27.09 for A; 27.87 for T; 16.89 for G; and 28.14% for C (GC content of 45%, and A + T content of 55%). The overall GC and AT content in A. farsicus was almost close to the fishes of family Cyprinodontidae such as Orestias ascotanensis, Jordanella floridae and Cyprinodon diabolis rather than the members of other families of the order Cyprinodontiformes (Table 5). These species are phylogenetically close to A. farsicus (Aphaniidae) rather than other examined fishes of the order Cyprinodontiformes (see phylogenetic tree).
Protein-coding Gene (PCG) Features
Aphanius farsicus mitochondrial genome encoded 13 protein-coding genes with 11462 bp in length, which accounted for 69.34% of the complete mitogenome (Table 5).
The boundaries between protein-coding genes of the mitochondrial genome were determined by aligning their sequences and identifying translation initiation and termination codons with comparison to other killifishes. The nucleotide composition in 13 protein coding genes was 25.40 for A, 30.27 for C, 15.43 for G and 28.88% for T. The A+T content of protein-coding gene were 61.20%. The lengths of protein-coding genes ranged in size from 169 (ATP8) to 1863 bp (ND5).
Transfer and Ribosomal RNA Gene Features
The complete mitochondrial genome in Aphanius farsicus contained 22 tRNA genes, which were totally 1552 bp in length and interspersed between the rRNA and the protein-coding genes with the ranges from 65 bp (tRNATry) to 77 bp (tRNASer and tRNAPro). The overall A + T content of 22 tRNAs was 57.20% with the composition of A = 30.21, G = 20.61, T = 26.99, C = 22.16%. The 16S and 12S ribosomal RNA genes were 1687 and 945 bp in length respectively, and located between genes tRNALeu and tRNAPhe and separated by gene tRNAVal. The A + T content was 55.80% for 16S rRNA gene (A = 32.36, G = 21.57, T = 23.41, C = 22.64%), and 52.20% for 12S rRNA gene A = 31.21, G = 21.26, T = 20.95, C = 26.56%), respectively.
Non-Coding Control Region (CR)
The nucleotide composition in D-loop (non-coding control region) was 28.95 for A, 23.19 for C, 15.61 for G and 26.58% for T. The A + T content of the control region were 61.20%, which was higher than the average value of the whole mitochondrial genome (i.e., 55.00%) (Table 4).
The mitochondrial DNA control region is a variable sequence that have a regulatory functional in transcription and replication of mtDNA. It is characterized by conserved sequence blocks, including the typical tripartition with a terminal associated sequence (TAS), a central and conserved sequence block (CSB) domains containing the conserved sequence blocks CSB-F, CSB-E and CSB-D (Sbisà et al., 1997). It additionally has a variable domain consists of three conserved sequence blocks (CSB-1, CSB-2, CSB-3). These blocks can be determined by multiple homologous sequence alignment with other related taxa.
The TAS domain is located between the 5' end of the control region and the beginning of the CSB-F. Generally, the length of TAS domain varied from 243 bp to 357 bp (Wang et al., 2011). Our results indicate that the TAS domain in A. farsicus was 301 bp length, and started with TACAT (Fig. 3). In addition, two central conserved sequence blocks CSB-F (TGAGACAAAAATCGTGGGGG) and CSB-D (TATTACTGGCATTTGGTTCCT) were detected in the control region of A. farsicus mitogenome (Fig. 3), while the typical central conserved CSB-E could not be found. Moreover, conserved sequence blocks; CSB-1, CSB-2 and CSB-3 were identified at the 3' end of the control region. These sequences might be associated in positioning RNA polymerase (Clayton, 1991; Shadel and Clayton, 1997).
Phylogenetic Analysis
Maximum likelihood tree was estimated based on whole mitochondrial genome of 16 examined killifish including Aphanius farsicus (Fig. 4). The ML tree presented seven major clades corresponding to the families of the order Cyprinodontiformes i.e., Rivulidae, Aplocheilidae, Poeciliidae, Fundulidae, Goodeidae, Cyprinodontidae, and Aphaniidae. Phylogenetic relationships based on the whole mitogenome of the examined killifishes are congruent with previous work (Quezada-Romegialli et al., 2015).
All the studied families are monophyletic. However, phylogenetic position of the clade VI (family Cyprinodontidae) and VII (family Aphaniidae) are questionable. In their previous study, Quezada-Romegialli et al. (2015) have documented high intra-family variability in the family Cyprinodontidae with regard to the whole mitogenome. They showed that within the family Cyprinodontidae, the genus Orestias is monophyly with high bootstrap value, and is sister to a clade containing Jordanella and Cyprinodon. The monophyly of genus Orestias and its phylogenetic relation to the genera Jordanella and Cyprinodon are now supported by our phylogenetic analysis (Fig. 4). However, they did not analyze genus Aphanius in their study.
Here for the first time we provided the complete mitogenome in one of the Aphanius species i.e., A. farsicus. In our phylogenetic hypothesis, we included the complete mitogenome of A. farsicus into the data set, and found that the genus Cyprinodon is sister to the genus Aphanius and both together form sister group to the genus Jordanella.
It should be noted that the genus Aphanius has already been in the family Cyprinodontidae. However, in their recent study, Freyhof et al. (2017) proposed the name Aphaniidae as valid family for the members of the genus Aphanius (the Western Palaearctic killifishes), while restricting the family Cyprinodontidae to the New World genera such as Cyprinodon, Floridichthys and Jordanella.
As mentioned above, in our phylogenetic analysis, a close phylgenetic relation was found between the genera Aphanius (now in the family Aphaniidae) and Cyprinodon (now in the family Cyprinodontidae) considering their complete mitogenome. Therefore, we assumed that the validation of family Aphaniidae still needs more phylogenetic supports, and this can be investigated by adding more sequences of the Aphanius members.
REFERENCES
Anderson, S., Bankier, A.T., Barrell, B.G., De Bruijn, M.H., Coulson, A.R., Drouin, J., Eperon, I.C., Nierlich, D.P., Roe, B.A., Sanger, F., Schreier, P.H., Smith, A.J., Staden, R., and Young, I.G., Sequence and organization of the human mitochondrial genome, Nature, 1981, vol. 290, pp. 457–465.
Angeletti, D., Cimmaruta, R., and Nascetti, G., Genetic diversity of the killifish Aphanius fasciatus paralleling the environmental changes of Tarquinia salterns habitat, Genetica, 2010, vol. 138, nos. 9–10, pp. 1011–1021. https://doi.org/10.1007/s10709-010-9487-3
Avise, J.C., Molecular Markers, Natural History and Evolution, Boston: Springer-Verlag, 1994.
Boore, J.L., Animal mitochondrial genomes, Nucleic Acids. Res., 1999, vol. 27, no. 8, pp. 1767–1780.
Coad, B.W., Distribution of Aphanius species in Iran, J. Am. Killifish Assoc., 2000, vol. 33, no. 6, pp. 183–191.
Coad, B.W., A new species of tooth-carp, Aphanius mesopotamicus, from Iran and Iraq (Actinopterygii, Cyprinodontidae), Zookeys, 2009, vol. 31, no. 131, pp. 149–163. https://doi.org/10.3897/zookeys.31.131
Edgar, R.C., MUSCLE: a multiple sequence alignment with high accuracy and high throughput, Nucleic Acids. Res., 2004, vol. 32, no. 5, pp. 1792–1797. https://doi.org/10.1093/nar/gkh340
Esmaeili, H.R., Teimori, A., Gholami, Z., and Reichenbacher, B., Two new species of the tooth-carp Aphanius (Teleostei: Cyprinodontidae) and the evolutionary history of the Iranian inland and inland-related Aphanius species, Zootaxa, 2014, vol. 3786, no. 3, pp. 246–268. https://doi.org/10.11646/zootaxa.3786.3.2
Esmaeili, H.R., Masoudi, M., Ebrahimi, M., and Elmi, A., Review of Aphanius farsicus: a critically endangered species (Teleostei: Cyprinodontidae) in Iran, Iran. J. Ichthyol., 2016, vol. 3, no. 1, pp. 1–18. https://doi.org/10.7508/iji.2016.01.001
Ferrito, V., Mannino, M.C., Pappalardo, A.M., and Tigano, C., Morphological variation among populations of Aphanius fasciatus Nardo, 1827 (Teleostei, Cyprinodontidae) from the Mediterranean, J. Fish. Biol., 2007, vol. 70, no. 1, pp. 1–20. https://doi.org/10.1111/j.1095-8649.2006.01192.x
Freyhof, J., Ozulug, M., and Sac, G., Neotype designation of Aphanius iconii, first reviser action to stabilize the usage of A. fontinalis and A. meridionalis and comments on the family group names of fishes placed in Cyprinodontidae (Teleostei: Cyprinodontiformes), Zootaxa, 2017, vol. 4294, no. 5, pp. 573–585. https://doi.org/10.11646/zootaxa.4294.5.6
Gholami, Z., Esmaeili, H.R., Erpenbeck, D., and Reichenbacher, B., Phylogenetic analysis of Aphanius from the endorheic Kor River Basin in the Zagros Mountains, Southwestern Iran (Teleostei: Cyprinodontiformes: Cyprinodontidae), J. Zool. Syst. Evol. Res., 2014, vol. 52, no. 2, pp. 130–141. https://doi.org/10.1111/jzs.12052
Gouy, M., Guindon, S., and Gascuel, O., SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building, Mol. Biol. Evol., 2010, vol. 27, no. 2, pp. 221–224.
Helmstetter, A.J., Papadopulos, A.S.T., Igea, J., van Dooren, T.J.M., Leroi, A.M., and Savolainen, V., Viviparity stimulates diversification in an order of fish, Nat. Commun., 2016, vol. 7, no. 11271, pp. 1–7. https://doi.org/10.1038/ncomms11271
Hrbek, T. and Meyer, A., Closing of the Tethys Sea and the phylogeny of Eurasian killifishes (Cyprinodontiformes: Cyprinodontidae), J. Evol. Biol., 2003, vol. 16, no. 1, pp. 17–36. https://doi.org/10.1046/j.1420-9101.2003.00475.x
Hrbek, T., Keivany, Y., and Coad, B.W., New species of Aphanius (Teleostei, Cyprinodontidae) from Isfahan Province of Iran and a reanalysis of other Iranian species, Copeia, 2006, vol. 2006, no. 2, pp. 244–255. https://doi.org/10.1643/0045-8511(2006)6[244:NSOATC]2.0.CO;2
Jondeung, A. and Karinthanyakit, W., Mitochondrial DNA control region of three mackerels, genus Rastrelliger: structure, molecular diversity and phylogenetic relationship, Mitochondrial DNA, Part A, 2015, vol. 27, no. 4, pp. 1–6. https://doi.org/10.3109/19401736.2015.1028047
Kim, Y., Kweon, H., Kim, I., Lee, Y., Kim, J., and Lee, J., The complete mitochondrial genome of the floating goby, Gymnogobius petschiliensis (Perciformes, Gobiidae), Mol. Cells, 2004, vol. 17, no. 3, pp. 446–453.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P., and Drummond, A., Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data, Bioinformatics, 2012, 28, no. 12, pp. 1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Keepers, K., Martin, A.P., and Kane, N.C., The complete mitochondrial genome of the Warm Springs pupfish, Cyprinodon nevadensis pectoralis, Mitochondrial DNA, Part A, 2016, vol. 27, no. 4, pp. 2349–2350. https://doi.org/10.3109/19401736.2015.1025259
Lema, S.C., Wilson, K.P., Senger, B.L., and Simons, L.H., Sequencing and characterization of the complete mitochondrial genome of the endangered Devils Hole pupfish Cyprinodon diabolis (Cyprinodontiformes: Cyprinodontidae), Mitochondrial DNA, Part B, 2016, vol. 1, no. 1, pp. 705–707.
Lowe, T.M. and Chan, P.P., tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes, Nucleic Acids Res., 2016, vol. 44, no. 1, pp. 54–57. https://doi.org/10.1093/nar/gkw413
Miya, M. and Nishida, M., Organization of the mitochondrial genome of a deep-sea fish, Gonostoma gracile (Teleostei: Stomiiformes): first example of transfer RNA gene rearrangements in bony fishes, Mar. Biotechnol., 1999, vol. 1, no. 5, pp. 416–426. https://doi.org/10.1007/PL00011798
Miya, M. and Nishida, M., Use of mitogenomic information in teleostean molecular phylogenetics: a tree-based exploration under the maximum-parsimony optimality criterion, Mol. Phylogenet. Evol., 2000, vol. 17, no. 3, pp. 437–455. https://doi.org/10.1006/mpev.2000.0839
Miya, M., Kawaguchi, A., and Nishida, M., Mitogenomic exploration of higher teleostean phylogenies: a case study for moderate-scale evolutionary genomics with 38 newly determined complete mitochondrial DNA sequences, Mol. Biol. Evol., 2001, vol. 18, no. 11, pp. 1993–2009. https://doi.org/10.1093/oxfordjournals.molbev.a003741
Nelson, J.S., Grande, T.C., and Wilson, M.V.H., Fishes of the World, Hoboken, NJ: Wiley, 2016.
Parenti, L.R., A phylogenetic and biogeographic analysis of cyprinodontiform fishes (Teleostei, Atherinomorpha), Bull. Am. Mus. Nat. Hist., 1981, vol. 168, pp. 335–557.
Pohl, M., Milvertz, F.C., Meyer, A., and Vences, M., Multigene phylogeny of cyprinodontiform fishes suggests continental radiations and a rogue taxon position of Pantanodon, Vertebr. Zool., 2015, vol. 65, no. 1, pp. 37–44.
Posada, D., jModelTest: Phylogenetic model averaging, Mol. Biol. Evol., 2008, vol. 25, no. 7, pp. 1253–1256. https://doi.org/10.1093/molbev/msn083
Quezada-Romegialli, C., Guerrero, C.J., Véliz, D., and Vila, I., The complete mitochondrial genome of the endemic and threatened killifish Orestias ascotanensis Parenti, 1984 (Cyprinodontiformes, Cyprinodontidae) from the High Andes, Mitochondrial DNA, Part A, 2016, vol. 27, no. 4, pp. 2798–2799. https://doi.org/10.3109/19401736.2015.1053072
Reznick, D.N., Furness, A.I., Meredith, R.W., and Springer, M.S., The origin and biogeographic diversification of fishes in the family Poeciliidae, PLoS One, 2017, vol. 12, no. 3, p. e0172546. https://doi.org/10.1371/journal.pone.0172546
Salimi, E., Zolgharnine, H., Archangi, B., Ronagh, M.T., and Ghasemi, S.A., Genetics diversity of Aphanius ginaonis and Aphanius dispar in Hormozgan and Bushehr coastal zones respectively using PCRRFLP molecular marker, J. Mar. Sci. Technol., 2018, vol. 17, no. 1, pp. 221–224.
Sbisa, E., Tanzariello, F., Reyes, A., Pesole, G., and Saccone, C., Mammalian mitochondrial D-loop region structural analysis: identification of new conserved sequences and their functional and evolutionary implications, Gene,1997, vol. 205, nos. 1–2, pp. 125–140. https://doi.org/10.1016/S0378-1119(97)00404-6
Setiamarga, D.H.E., Miya, M., Yamanoue, Y., Mabuchi, K., Satoh, T.P., Inoue, J.G., and Nishida, M., Interrelationships of Atherinomorpha (medakas, flyingfishes, killifishes, silversides, and their relatives): the first evidence based on whole mitogenome sequences, Mol. Phylogenet. Evol., 2008, vol. 49, no. 2, pp. 598–605. https://doi.org/10.1016/j.ympev.2008.08.008
Stamatakis, A., RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models, Bioinformatics, 2006, vol. 22, no. 21, pp. 2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Teimori, A., The evolutionary history and taxonomy of Aphanius (Teleostei: Cyprinodontidae) species in Iran and the Persian Gulf region, PhD Thesis, Munich: Ludwig-Maximilians Univ., 2013.
Teimori, A., Esmaeili, H.R., and Reichenbacher, B., Aphanius farsicus, a replacement name for A. persicus (Jenkins, 1910) (Teleostei, Cyprinodontidae), Zootaxa 2011, vol. 3096, pp. 53–58. https://doi.org/10.11646/zootaxa.3096.1.5
Teimori, A., Esmaeili, H.R., Gholami, Z., Zarei, N., and Reichenbacher, B., Aphanius arakensis, a new species of tooth-carp (Actinopterygii, Cyprinodontidae) from the endorheic Namak Lake basin in Iran, Zookeys, 2012, vol. 215, no. 1731, pp. 55–76. https://doi.org/10.3897/zookeys.215.1731
Teimori, A., Esmaeili, H.R., Erpenbeck, D., and Reichenbacher, B., A new and unique species of the genus Aphanius (Teleostei: Cyprinodontidae) from Southern Iran: a case of regressive evolution, Zool. Anz., 2014, vol. 253, no. 4, pp. 327–337. https://doi.org/10.1016/j.jcz.2013.12.001
Teimori, A., Mostafavi, H., and Esmaeili, H.R., An update note on diversity and conservation of the endemic fishes in Iranian inland waters, Turk. J. Zool., 2016, vol. 40, no. 1, pp. 87–102. https://doi.org/10.3906/zoo-1407-2
Teimori, A, Esmaeili, H.R., Hamidan, N., and Reichenbacher, B., Systematics and historical biogeography of the Aphanius dispar species group (Teleostei: Aphaniidae) and description of a new species from Southern Iran, J. Zool. Syst. Evol. Res., 2018, vol. 56, no. 4, pp. 579–598. https://doi.org/10.1111/jzs.12228
van der Laan, R., and Fricke, R., Family-group names, 2018, Version 12/2018. http://www.calacademy.org/scientists/catalog-of-fishes-family-group-names/.
Villwock, W., A contribution to the understanding of the evolution of meristic characters, with special reference to Old World Cyprinodontids (Pisces, Cyprinodontidae), Abh. Ver. Naturwiss. Ver. Hamb, 1976, vol. 18–19, pp. 11–27.
Wildekamp, R.H., A World of Killies: Atlas of the Oviparous Cyprinodontiform Fishes of the World, Houston: Am. Killifish Assoc., 1993.
Xu, L., Wang, X., Li, H., and Du, F., The complete mitochondrial genome of Perciformes fish (Brama dussumieri) from South China Sea, Mitochondrial DNA, Part B, 2018, vol. 3, no. 2, pp. 874–875. https://doi.org/10.3390/ijms19061741
Yaripour, S., Esmaeili, H.R., Gholamhosseini, A., Rezaei, M., and Sadeghi, S., Assessment of genetic diversity of an endangered tooth-carp, Aphanius farsicus (Teleostei: Cyprinodontiformes: Cyprinodontidae) using microsatellite markers, Mol. Biol. Res. Commun., 2017, vol. 6, no. 4, pp. 153–160. https://doi.org/10.22099/MBRC.2017.24404.1246
Zhang, X.Y., Yue, B.S., Jiang, W.X., and Song, Z., The complete mitochondrial genome of rock carp Procypris rabaudi (Cypriniformes: Cyprinidae) and phylogenetic implications, Mol. Biol. Rep., 2009, vol. 36, no. 981, pp. 981–991. https://doi.org/10.1007/s11033-008-9271-y
ACKNOWLEDGMENTS
The authors are grateful to A. Khajooei for her assistance with laboratory experiments, and M. Ebrahimi for giving the permission of A. farsicus photo to be used in this study.
Funding
This research was funded by support from the Iran National Science Foundation (grant no. 96000798).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Teimori, A., Motamedi, M. The First Complete Mitochondrial Genome Sequence in the Genus Aphanius (Teleostei). J. Ichthyol. 59, 754–765 (2019). https://doi.org/10.1134/S0032945219050151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945219050151