Abstract
Complex ecosystem analysis which includes total abundance estimation of its members as well as their quantitative trophic relationships gives an insight to ecosystem structure, its dynamic and steadiness and allows to compare large marine ecosystems. In this paper we aim to reveal and quantify the seasonal dynamics of main trophic web components and to estimate nekton (fish and squids) food capacity throughout the summer-autumn in upper epipelagic layer of deep basins in the Western Bering Sea. We used nekton and plankton abundance data as well as nekton diet data obtained in June-October throughout 2003–2015. Log-transformed abundance were averaged by 10-day periods considering the probability of occurrence of given species. We have found that the total biomass of nekton and the species ratio showed severe changes within summer-autumn. Total biomass reached the maximum in August showing two- or three-time increase from early-summer and followed by the similar decrease in autumn. The total food consumption changed in the same way. However, the grazing pressure on prey was very low as the consumer/prey biomass ratio approached zero. In addition, we have recognized diet divergence both between species and within size group of one specie. We conclude that the spatio-temporal separation of mass nekton species and differences in their diet lead to alleviation of trophic competition and grazing pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Trophological studies of marine and oceanic ecosystems are becoming more and more urgent as understanding the structure of trophic networks not only reveals the principal traits of marine communities' functioning under various conditions of their existence but also gives grounds to estimate the potential restructuring under various environmental factors.
During long-term investigations in the Western Bering Sea, a solid data array has been collected on food resources and feeding of nekton. Such considerable information allowed to determine the role of mass species of fish and squids (Teuthida) in the trophic structure of epipelagic communities and to estimate their food supply influence on food resources in different periods (Shuntov et al., 1993, 2010; Chuchukalo, 2006; Naydenko, 2007, 2009, 2010; Shuntov and Temnykh, 2008a, 2008b, 2011; Zavolokin, 2011, 2014; Shuntov, 2016). In addition, Ecopath model applied to the Western Bering Sea (Aydin et al., 2002; Radchenko, 2011, 2015) defined principal functional parameters of the ecosystem. Impact of Pacific salmon abundance on food capacity of the other nektonic species was also modeled (Zavolokin et al., 2014, 2016). At the same time, some studies (Klovach, 2003; Karpenko et al., 2013) assume earlier (Birman, 1985) ideas that being very abundant in marine habitat, salmons experience severe intra- and interspecific competition for food resources. Consequently, insufficient food capacity affects biological conditions and abundance of Pacific salmon.
The present article is a continuation of studies (Naydenko et al., 2007a, 2007b; Naydenko, 2009; Zavolokin, 2011; Zavolokin et al., 2014, 2016) on trophodynamics of pelagic communities in the Bering Sea. The present study aims to reveal and quantify the seasonal dynamics of main trophic web components and to estimate nekton food capacity throughout summer-autumn in upper epipelagic layer of deep basins in the Western Bering Sea.
MATERIALS AND METHODS
The study is based on data on the composition and abundance of plankton and nekton as well as on the diet of nekton collected in 17 surveys conducted by TINRO-Center in the Western Bering Sea in June–October 2003–2015. Information on plankton and nekton was averaged by two standard biostatistic regions (the Commander and the western part of the Aleutian basins (Fig. 1)) specified with consideration for the general scheme of water circulation, bottom relief, and distribution of water masses (Shuntov et al., 1986, 1988a, 1988b; Volvenko, 1998, 2003; Nekton…, 2006).
Nekton was caught by trawling in the upper epipelagic layer (0–30 m) using an RT 80/396 midwater trawl (average vertical aperture 33.7 m, horizontal aperture 39 m, 10 mm mesh is inserted in the codend). Averaged technical parameters of trawling were the following: trawling time 60 min, headrope horizon 0 m, trawling speed 4.6 knots, wires length 270.6 m. Altogether, 1098 trawlings were done. Trawl catches were sorted by species. Species names of fish were verified via the Eshmayer catalogue (Catalog of Fishes..., 2018), and names of squids are given according to CephBase (http://cephbase.eol.org. Version 2018).
Hydrobionts were counted, weighted and measured (fork length for fish (FL) and mantle length for squids). Salmons and other commercial species were fully analyzed (individual weight, sex, maturity stage). Relative abundance (n, ind./km2) and biomass (m, kg/km2) of each species at each trawl station were calculated with consideration for the trawled area (Aksyutina, 1968) applying catchability coefficients for each nekton species (Volvenko, 1998). Full descriptions of abundance calculation can be found in the tabulated bulletins of TINRO-Center (Nekton…, 2006; Makrofauna…, 2012). The data on abundance of hydrobionts is lognormally distributed, therefore logarithms were taken to correct calculation of average values. Mean abundance (N, ind./km2) and biomass (B, kg/km2) were calculated as a mean for all trawlings (where a particular species occurred) multiplied by the probability of occurrence of this particular species to be caught within a particular region or a time period:
where n and m are abundance (ind./km2) and biomass (kg/km2) of the species in the catch where this species occurred; P is probability of occurrence of the species calculated by the number of trawlings where this species occurred (Tp) to the total number of trawlings (Tt): P = Tp/Tt.
The values of abundance for species, which undertake diel vertical migrations and occur in catches only at night (boreopacific gonate squid Boreoteuthis borealis, northern lumpfish Stenobrachius leucopsarus, northern smoothtongue Leuroglossus schmidti, scaly paperbone Scopelosaurus harryi, and eared blacksmelt Lipolagus ochotensis) are calculated by considering night trawlings only. Seasonal dynamics of composition and abundance of nekton for the period from the beginning of June to the end of October was analyzed by data averaged for 10-day periods. Methods of calculation of species abundance has been described previously (Somov, 2017).
Plankton was captured by a large Juday net, aperture area 0.1 m2, with caprone sieve no. 48 (0.168 mm mesh size). Plankton sampling spatially overlapped trawl and hydrological stations. Samples were taken from 2 layers (50–0 m and 200–0 m). Sample processing and abundance calculations were done aboard according to Volkov (1996, 2008). Each sample was subdivided into three fractions: small-sized, medium-sized, and large (<1.2, 1.2–3.2, and >3.2 mm). The plankters were identified to the species level. Abundance and biomass were calculated for each fraction of each sample using catchability coefficients (Volkov, 1996, 2008). Plankters’ abundance and biomass were averaged for biostatistical regions (the same as for nekton). Data from TINRO-Center’s Net Zooplankton Database (Volvenko, 2016) was used to quantify seasonal changes of zooplankton abundance. Diet analysis was also done aboard according to standard express processing methods developed in TINRO-Center (Rukovodstvo…, 1986; Chuchukalo, 2006; Volkov, 2008). Daily food rations (R, % of body weight) of fish and squids were calculated by various methods (Romanova, 1958; Kogan, 1963; Chuchukalo, 1996; Volkov, 2008) with consideration for each species’ diet strategy. Cluster analysis (Ward dendrogram) was applied to elucidate similarity in feeding habits of mass nekton species.
To estimate feeding condition for nekton, we assessed their consumption of each prey using the following equation: Mj = Bi × R × t, where Mj is weight of the consumed food item (of j-th prey), tn; Bi is biomass of the consumer (of an ith predator), tn; R is daily ration, % of the body weight; t is period, days. Summing up the consumption values of all prey, we calculated total consumption of given nekton species or group of species. Total food consumption was calculated per 1 day (24 h). To compare total grazing pressure on prey between biostatistical regions, we standardized data to area relative values (kg/km2 day–1).
An energy transport from lower trophic level to higher ones is carried out by consuming producer organisms by primary consumers, and further up to the highest trophic levels. This translocation is not direct but distributed between many species and results in energy flows. The scope of this study concentrates on energy flows within zooplankton-nekton community. Herewith not only do nekton species consume zooplankton but also large nekton individuals feed small ones. The small-sized nekton comprised the following: juveniles of fish (walleye pollock Theragra chalcogramma, Atka mackerel Pleurogrammus monopterygius, and other bottom fish), small fish (three-spined stickleback Gasterosteus aculeatus, northern lumpfish, northern smoothtongue, eared blacksmelt, etc.), and small individuals as well as juveniles of squid. All nektonic species occurring in the food of fish and squids are united into “nektonic food” group.
The level of grazing pressure was estimated comparing the total consumption by nekton with total prey abundance in the epipelagic layer. This coefficient is called forage base usage. The closer is this parameter to unity, the higher is the nekton press on the food resources and the lower is its food capacity (Zheltenkova, 1955a, 1955b; Chuchukalo, 2006). This coefficient may be calculated as diurnal, monthly, seasonal, or annual.
Trophic levels (TL) of plankton and nekton species were calculated in the TrophLab application (June 2000 version) by the following equation:
where DCij is fraction of jth prey in the diet of ith consumer, TLj is mean trophic level of each jth prey, and G is number of preys in the diet of ith consumer (Gascuel and Pauly, 2009).
To visualize trophic webs we used Gephi 0.8.2 (gephi.org). On the schemes, the “other fish” group comprises the species having a low occurrence and a low biomass in catches and the “juvenile squid” group comprises individuals with the mantle length less than 4 cm. The total number of trophic links of the form “prey → consumer” demonstrates the ramified structure of the trophic web. Given the consuming rate, each trophic link were specified as primary (≥0.5 kg/km2) or secondary (<0.5 kg/km2) one. The total value of principal links is equal to the sum of particular trophic interrelations (expressed by weight ≥0.5 kg/km2 day) between the food item and its consumer (a nekton species). We used the proportion of primary trophic links to compare ramified structure of the trophic web between seasons and regions. Another type of trophic link was cyclic. It occurred in case of cannibalism which is very important for walleye pollock and squids (Volkov et al., 1990; Chuchukalo, 2006; Volkov, 2015).
RESULTS AND DISCUSSION
In June–October 2003–2015, 97 species of fish and squids belonging to 42 families were identified in catches in the upper epipelagial of the Bering Sea. By the number of species, the most abundant were Gonatidae (15 species), Cottidae (eight), Pleuronectidae (eight), Salmonidae (six), Gadidae (five), and Myctophidae (four). Other families comprised not more than three species, frequently just one species (Somov, 2017). There are considerable seasonal changes in the species composition and abundance (Nekton…, 2006; Macrofauna…, 2012; Shuntov, 2016). Depending on migration periods of mass species of fish and squids, three periods were discerned: early summer—from the first 10-day period of June until the second 10-day period of July; summer period—from the third 10-day period of July until the second 10-day period of September; autumn period—from the third 10-day period of September until October (Somov, 2017).
The early summer period. In June–early July, mature pink salmon Oncorhynchus gorbuscha and mature chum salmon O. keta intensively migrates across the deep basins toward the spawning areas located in Karaginsk–Olyutorsk and Koryak–Anadyr regions. Immature chum salmons begin the feeding migration in this period (Radchenko et al., 1995; Starovoytov, 2003a; Shuntov and Temnykh, 2008b, 2011; Shuntov, 2016). Average biomass of pink salmon in the upper epipelagial of the Commander Basin and in the western part of the Aleutian Basin for the early-summer was 175 and 133 kg/km2, respectively; biomass of chum salmon in these regions was higher: 227 and 393 kg/km2; abundance of other salmon species were lower (Fig. 2). In chum salmon, the large mature (FL > 50 cm) specimens and immature ones (FL 40–50 and > 50 cm) individuals (Fig. 3). In addition to salmons, the abundance of squids was noticeable, mainly of boreopacific gonate squid and of juveniles of various species of the family Gonatidae (Fig. 2). In the years of high abundance, being neretic species, herring Clupea pallasii of the Korf-Karagin population may advance in open deep-sea waters (Shuntov et al., 1988b; Naumenko, 2010; Shuntov, 2016). We detected this phenomenon several times in our study.
Composition and biomass of nekton in the upper epipelagial of the Commander Basin and in the western part of the Aleutian Basin of the Bering Sea in different seasons of the 2000s: ( ) chum salmon Oncorhynchus keta, (
) chum salmon Oncorhynchus keta, ( ) pink salmon O.gorbuscha, (
) pink salmon O.gorbuscha, ( ) sockeye salmon O. nerka, (
) sockeye salmon O. nerka, ( ) other salmon species, (
) other salmon species, ( ) other fish, (
) other fish, ( ) squids (Teuthida).
) squids (Teuthida).
Abundance of the aforelisted nekton species controlled the direction and value of organic matter flow in the upper epipelagic foraging food web of Western Bering Sea in the early summer period. Mean long-term value of food consumption by fish and squids in this period in the upper layer of the epipelagial of the Commander Basin is estimated as 22 kg/km2 per day and 25 kg/km2 per day in the western part of the Aleutian Basin (Fig. 4). In both regions, the food consumption by large-sized chum salmon, pink salmon, and squids (principally by boreopacific gonate squid) was considerable. Diel food consumption by pink and chum salmons attained 80% of all food consumed by nekton in the upper epipelagial. Mean long-term proportion of squids in the diel consumption of resources in the Commander Basin was 14% due to boreopacific gonate squid and juveniles of the family Gonatidae being very abundant in this region. In the western part of the Aleutian Basin, the food consumption by squids was lower. Thus, in the upper epipelagial of these regions, the bulk of food resources was consumed at trophic levels 3.5–3.8, and the principal consumers were pink salmon, large-sized mature chum salmon, and boreopacific gonate squid (Fig. 5).
Organic matter is transferred from the primary to upper trophic levels via zooplanktonic organisms. In both regions, the main prey were euphausiids (Euphausiacea, principally Thysanoessa longipes), pteropods (Pteropoda, Limacina helicina and Clione limacina), amphipods (Amphipoda, mainly Themisto pacifica), and jellyfish (Jellyfish, Ctenophora, Oikopleura, etc.), while along with those in the Commander Basin were also copepods (Copepoda, Neocalanus cristatus and Eucalanus bungii) (Fig. 4). The fraction of the aforesaid food groups consisted > 80% of all food consumed by nekton in the upper layer of the epipelagial. Euphausiids were consumed mainly by mature pink salmon, large-sized mature chum salmon, and by boreopacific gonate squid. Just these three nekton species consumed 7.7 and 6.6 kg/km2 euphausiids per day in the Commander Basin and in the western part of the Aleutian Basin respectively. Consumption of euphausiids by all species of nekton in these regions was estimated as 9.0 and 7.8 kg/km2 per day, respectively. In the western part of the Aleutian Basin, where the biomass of feeding chum salmon of various size was high (Fig. 3), considerable quantities of pteropods and jellyfish were consumed: 6.1 and 2.9 kg/km2 per day, respectively. Specialization on pteropods and trophic selectivity of chum salmon in relation to jellyfish was previously noted (Volkov, 1994; Volkov et al., 1997; Dulepova, 1998; Starovoytov, 2003b; Chuchukalo, 2006; Zavolokin, 2008). The next group of zooplankton clearly selected by salmons was amphipods (hyperiids) (Volkov, 1996; Gorbatenko, 1996; Chuchukalo, 2006; Zavolokin, 2008; Naydenko et al., 2008). In the western part of the Aleutian Basin, the salmons consumed 2.6 kg/km2 of hyperiids per day, while other nekton groups comprised only 0.2 kg/km2 per day. In the Commander Basin, the abundance of salmons was lower and they consumed less amphipods: 1.5 (other nekton species consumed 0.3) kg/km2 per day. Copepods were present in the diet of many fish and squid species. Copepods usually consist the bulk of food in Atka mackerel, walleye pollock, herring, and mesopelagic fish (Efimkin, 2006; Chuchukalo, 2006) whose biomass was insignificant in both regions (Fig. 2). Therefore, grazing of copepods by nekton made just 1.2 and 0.8 kg/km2 per day in the Commander Basin and in the western part of the Aleutian Basin, respectively. The fraction of nektonic food mainly consumed by fish and squid made 15–19% of the total food resources (Fig. 4).
The aforementioned food items formed the principal trophic interrelations of nekton in the early summer period. All other prey occurring in the consumers of high trophic level diet were secondary or occasional food items contributing to the diversity of trophic interrelations. The total number of trophic links in the form of “prey → consumer” (a nektonic species) in the Commander Basin was 282, while that in the Aleutian Basin was lower: 202. A more ramified trophic web in the upper epipelagial of the Commander Basin depends on a more diverse composition of consumers foraging in this region. Since the biomass of principal consumers in both regions differed insignificantly, the proportion of primary trophic links was at almost the same level: 70 and 74% of all consumed food in the Commander Basin and in the western part of the Aleutian Basin, respectively (Table 1).
The summer period. As the summer period develops, the biomass of nekton in the upper epipelagial of the Commander Basin increased by 1.6 times and that in the western part of the Aleutian Basin increased by almost three times (Fig. 2). This was mainly due to massive feeding migration of immature chum salmon (1 marine year and older), sockeye salmon O. nerka, and Chinook salmon O. tshawytscha from adjacent areas. In the Commander Basin, the specific biomass of immature feeding chum salmon (FL > 40 cm) was 350 kg/km2 and that in the Aleutian Basin was 490 kg/km2 (Figs. 3a, 3b). It should be noted that the abundance of mature chum salmon FL 40–50 cm in this region was also high (353 kg/km2). The biomass of large immature sockeye salmon in the Commander Basin and in the western part of the Aleutian Basin increased by 3 and 19 times, respectively, while that of chinook salmon increased by eight and four times, respectively. On the contrary, biomass of pink salmon in both regions decreased as they finish the anadromous migration through deep basin and already entered the coast waters and further enter the rivers moving upstream (Shuntov and Temnykh, 2008b, 2011). Of other nekton species, the squid biomass increased: two times in the Commander Basin and seven times in the western part of the Aleutian Basin (Fig. 2).
Compared with June, the daily consumption of food by nekton in the upper layer of the epipelagial increased in the Commander Basin and in the western part of the Aleutian Basin by 1.6 times (to 35.7 kg/km2 per day) and 3.0 times (to 74.4 kg/km2 per day) on average, respectively (Fig. 4). Mean salmon proportinon in the general food consumption in the Commander Basin and in the western part of the Aleutian Basin was 79 and 87%, respectively, while that of squids was 16 and 9%. Thus, in summer in the upper epipelagial of investigated regions, major flows of organic matter onto upper trophic levels 3.0–4.0 were mostly directed to salmons (chum salmon and sockeye salmon) and in less degree to squids (Fig. 6).
Schemes of nekton’s trophic interrelations in the upper epipelagial of (а) the Commander Basin and (b) the western part of the Aleutian Basin in summer of the 2000s; see designations in Fig. 5.
In the Commander Basin, due to increased biomass of immature feeding chum salmon and sockeye salmon, the consumption of amphipods, pteropods, and small-sized nekton increased (Fig. 4). The consumption of euphausiids changed insignificantly as the biomass of some consumers of euphausiids, in particular squids, increased while other main consumers of euphausiids decreased (pink salmon). In the Aleutian Basin, the consumption of euphausiids and of small-sized nekton, amphipods, and copepods significantly increased due to active feeding on these hydrobionts by foraging juvenile salmons of various-sizes and consumption by squids. The consumption of jellyfish decreased in the Commander Basin while increased in the Aleutian Basin due to changing abundance and diet of chum salmon-main consumer of jellyfish.
The change in consumption of certain prey is related not only to abundance of consumers and to ecology of their feeding (Fig. 7). In some cases, there was simultaneous increase (or decrease) of prey in the diet proportion and in the total abundance. In the end of summer and in autumn, the biomass of amphipods increased by three to four times in the upper epipelagial of the deep-sea (Figs. 7c, 7d, 7g, 7h). It is supposed that the increase in the abundance is related to growth and development of the summer generation of crustaceans and to the beginning of appearance juveniles of mass species of hyperiids from the autumn generation (Shebanova et a., 2014). In the same period, the fraction of hyperiids in the diet of nekton increased, e.g., in the ration of chum salmon of all size groups in both regions (Figs. 8a, 8b) and in the diet of sockeye salmon (Figs. 8с, 8d) and of Atka mackerel in the Commander Basin. In the summer period, the biomass of euphausiids also increased, reaching maximum values in August–September but followed by decreasing in October–November (Figs. 7a, 7b, 7e, 7f). This is related to seasonal vertical redistribution of various size groups of these crustaceans and to their consumption (Chuchukalo et al., 1996; Shuntov, 2001). Within the study area, the euphausiids were consumed by upper trophic levels throughout summer and autumn. In many species, including salmons, the proportion of euphausiids in the diet decreased in the summer period and, especially, in autumn (Fig. 8). The changes in the composition and abundance of small-sized nekton were also reflected in the food composition of fish and squids. In deep-sea basins of the Bering Sea the following species are mainly dominant among small-sized nekton: northern lumpfish, northern smoothtongue, juvenile Alka mackerel, juvenile squids, and three-spined stickleback in some years (Zavolokin, 2008). In summer juveniles of Atka mackerel are massively drifted in the upper epipelagial of Western Bering Sea deep basins (Mel’nikov and Efimkin, 2003). Biomass of juvenile Atka mackerel in the deep-sea regions increased in August–September by almost 30 times (Fig. 9). In this period, the juveniles of Atka mackerel were actively consumed by salmons. For example, chum salmon, chinook salmon, and sockeye salmon consumed up to 6 kg/km2 of juvenile Atka mackerel per day in the western part of the Aleutian Basin. At the same time in the Commander Basin, where juvenile Atka mackerel was much more abundant, their consumption was only 0.6 kg/km2 per day due to lower biomass of salmons. After spawning period in deep layers newly-emerged squid juveniles (mainly boreopacific gonatr squid) passively migrate in upper epipelagic layer. Mean specific biomass of juvenile squids in summer is estimated as 60 kg/km2 in the Commander Basin (Fig. 9a) and as 52 kg/km2 in the western part of the Aleutian Basin (Fig. 9b). Their proportion in the diet of salmons, especially of sockeye salmon (Figs. 8c, 8d), and in the diet of adult squid also increased. Generally, the proportion of nektonic food in the diet of fish and squid increased by 2–3 times, i.e., some redistribution of load on prey groups took place. It should also be noted that in summer the trophic level of salmons and squids increased up to 4.0–4.1 due to the decreased fraction of zooplanktonic food and the increased fraction of nekton food in the diet of chum salmon and sockeye salmon and the increased fraction of squid juveniles in the diet of adult squids (Fig. 6).
Seasonal dynamics of various groups of zooplankton’s biomass in the epipelagial of (а, с, e, g) the Commander Basin and (b, d, f, h) in the western part of the Aleutian Basin of the Bering Sea within (a–d) 0–50 m and (e–h) 0–200 m depth ranges in 1986–2015 (Netted Zooplankton of the North Pacific in 1984–2013 database of TINRO-Center): (a–b, e–f) ( ) small-sized fraction, (
) small-sized fraction, ( ) middle-sized fraction, (
) middle-sized fraction, ( ) Copepoda, (
) Copepoda, ( ) Chaetognatha; (c–d, g–h) (
) Chaetognatha; (c–d, g–h) ( ) Amphipoda, (
) Amphipoda, ( ) Euphausiacea.
) Euphausiacea.
Seasonal dynamics of biomass of small-sized nekton in the upper epipelagial of (а) the Commander Basin and (b) the western part of the Aleutian Basin of the Bering Sea in the 2000s: ( ) juvenile Boreopacific gonate squid Boreoteuthis borealis, (
) juvenile Boreopacific gonate squid Boreoteuthis borealis, ( ) juvenile squids (Teuthida), (
) juvenile squids (Teuthida), ( ) juvenile Atka mackerel Pleurogrammus monopterygius, (
) juvenile Atka mackerel Pleurogrammus monopterygius, ( ) mesopelagic fish, (
) mesopelagic fish, ( ) juveniles of other fish.
) juveniles of other fish.
In spite of the increased biomass of consumers, the total number of trophic links in the trophic web of the upper layer of the epipelagial in the Commander Basin decreased in comparison with that in the early summer by 1.8 times, while that in the Aleutian Basin changed insignificantly. However, the number of primary links increased in both regions and their proportion of the total consumption by nekton was over 80% (Table 1, Fig. 6). It is known that the total number of food items (or of trophic interrelations) usually decrease at rich food resources (Nikolskii, 1953; Zheltenkova, 1973). In the summer period, the increase in biomass of consumers and of consumed volumes of food resources were recorded, but no increase in the total number of trophic interrelations or in the fraction of secondary interrelations were recorded, which might indicate to deterioration of foraging conditions.
The autumn period. From September until November the abundance of middle-sized and large-sized salmons decreases in deep-sea regions. At the same time, their biomass, in particular that of chum salmon and sockeye salmon, still remains significant. Biomass of small-sized juveniles (especially that of pink salmon) increases as they advance from coastal areas offshore. Preparing to the following winter, all salmons start migrate toward Southern Bering Sea and Aleutian waters of the North Pacific (Radchenko, 1994; Starovoitov, 2003a; Glebov, 2007a, 2007b, 2007c; Shuntov and Temnykh, 2008b, 2011; Shuntov, 2016). Among other nekton species, the biomass of squids increases (mainly of boreopacific gonate squid), especially in the western part of the Aleutian Basin (Fig. 2) (Starovoytov, 2003a; Glebov, 2007a, 2007b, 2007c; Somov, 2017). The boreopacific gonate squid is an active vertical migrant forming high concentrations in the dark period of the day in the upper layer of the epipelagial (Nesis, 1997; Watanabe et al., 2006; Zuev et al., 2007). After summer and summer–autumn spawning, the juveniles of this species appear in abundance in the upper epipelagial (Zuev et al., 2007). Biomass of juveniles of boreopacific gonate squid in autumn in the Commander Basin and in the western part of the Aleutian Basin was 69 and 151 kg/km2, respectively (Figs. 9a, 9b). The walleye pollock is also important in the trophic structure of the pelagial of the Bering Sea (not only in shelf and slope areas but also in deep-sea basins), especially in autumn (Shuntov et al., 1993; Naydenko, 2007; Zavolokin et al., 2014). In different years, the abundance of walleye pollock in deep-sea areas considerably varies depending on variation of the general biomass of populations, abundance of particular generations, oceanological conditions, etc. (Shuntov et al., 1993; Stepanenko, 1997, 2001; Shuntov, 2016). In recent decades after decrease of its abundance, the distribution of walleye pollock of all stocks into deep-sea basins decreased considerably (Shuntov, 2016). In the 2000s in the upper layer of the epipelagial of the deep-sea basins, the mean long-term specific biomass of walleye pollock calculated by the data of trawl catches did not surpass 5.0–7.0 kg/km2. One more important element of the trophic structure of the upper epipelagial of deep-sea regions in autumn is the three-spined stickleback. This species is characterized by noticeable periodical increase of abundance (Chereshnev et al., 2002; Bugaev et al., 2007; Starovoytov et al., 2014). Stickleback fry conduct their seaward migration from Kamchatka fresh water bodies in August–September and mass aggregations are mainly caught in the Commander Basin along the slope of Olyutorsk Bay and Karaginsk Bay. Biomass of stickleback in this region in late September and early October was 2.5 and 1.5 kg/km2, respectively. In late summer–early autumn species belonging to low boreal-subtropical complex: Japanese anchovy Engraulis japonicus, saury Cololabis saira, Pacific pomfret Brama japonica may migrate into the deep-basin regions of Western Bering Sea (Radchenko, 1994; Glebov et al., 2010; Shuntov, 2016). In the period of investigations, their proportion, mainly that of saury, did not surpass 1.5% of the total biomass of nekton.
Due to autumn restructuring in the composition and abundance of mass species of the nekton community, the consumption of food by nekton decreased two times on average in comparison with summer: to 22 kg/km2 per day in the Commander Basin and to 33 kg/ km2 per day in the western part of the Aleutian Basin (Table 1). In both regions, food consumption by large-sized chum salmon and sockeye salmon decreased rather significantly. At the same time in the Commander Basin, the food consumption by migrating juveniles of salmons increased, in particular by pink salmon. Food consumption by boreopacific gonate squid also increased: by 1.8 times (to 7.4 kg/km2 per day) in the Commander Basin and by 3.3 times (to 15.6 kg/km2 per day) in the western part of the Aleutian Basin. Thus, the principal consumers of food resources in the upper epipelagial in both regions were boreopacific gonate squid, chum salmon, juvenile pink salmon, and sockeye salmon (Fig. 10). The proportion of these species in the total food consumption by nekton were 88 and 95% in the Commander Basin and in the western part of the Aleutian Basin, respectively. Other consumers may be considered as secondary. In the Commander Basin, these are Atka mackerel, Chinook salmon, saury (mainly in the south of this region), northern smoothtongue, capelin Mallotus villosus, three-spined stickleback, and squid. The total fraction of these species was ~7% of the total food consumption by nekton. In the Aleutian Basin, the fraction of secondary food consumers (Chinook salmon, herring, Atka mackerel, northern smoothtongue, and squid) is estimated as 4% of the total food consumption. The food consumption by walleye pollock recorded in the upper layer of the epipelagial did not exceed 0.1 kg/km2 per day throughout summer–autumn. It should be noted that walleye pollock in the years of its high abundance was one of the principal elements of the trophic web, mainly in the late summer and in autumn in shelf and slope regions (Naydenko, 2007).
Schemes of trophic interrelations in the upper epipelagial of (а) the Commander Basin and (b) the western part of the Aleutian Basin in autumn of the 2000s; see designations in Fig. 5.
The energy flow in the Commander Basin were carried out through ampipods (T. pacifica), euphausiids (Th. longipes and less so Th. inermis), pteropods (principally С. limacina), and various species of small-sized nekton. The proportion of amphipods in the total food consumption increased to 30% due to their active consumption by juvenile salmons and squid. In the western part of the Aleutian Basin, the major food items were euphausiids, pteropods, amphipods, and small-sized nekton (Fig. 4). These trophic groups made 82 and 93% of the total food consumed by nekton in the Commander Basin and in the western part of the Aleutian Basin, respectively. The total number of trophic links of nekton decreased only in the western part of the Aleutian Basin, the number of primary trophic links decreased in both deep-sea regions. It happened due to decrease in abundance of such consumers as chum salmon and sockeye salmon and beginning of their seasonal migration to the North Pacific. The proportion of primary trophic links in the total diel food consumption by nekton did not change (Table 1).
Thus, due to changes in composition and abundance of main consumers, total consumption by fish and squid in the upper epipelagic layer of Western Bering Sea deep regions decreased. Squid and salmons remained major consumers of food resources. In the Commander Basin, the fraction of interrelationships amphipods → salmons and amphipods → squids increased in the total food consumption.
CONCLUSIONS
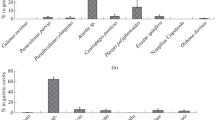
The seasonal changes in the trophic structure of nektonic communities of the upper epipelagial of the western part of the Bering Sea depend on various reasons and are characterized by specific conditions for this region. First, there is a considerable difference in the forage period and region of salmons (mature, large-sized, and medium-sized, and juveniles), squid (juveniles and adults), Atka mackerel, walleye pollock, and herring (Figs. 2, 3, 9). Cooccurrence in deep-sea regions of these mass species of nekton (and of their size-age groups) would increase the pressure on food resources by several times. Secondly, feeding selectivity and presence of cyclic interrelationships in some nektonic species lead to a considerable divergence of food spectra in mass species of fish and squid. Cluster analysis showed that the food composition differs not only between different species but also within size groups of these species (Fig. 11). Third, the seasonal changes occurring in the composition and abundance of food organisms significantly expand the forage base of consumers due to the possibility to feed on not only preferred but on mass available prey (in particular, on juveniles of fish and squid). The listed traits decrease the food competition between consumers and result in a more homogeneous grazing pressure distribution on food resources.
Comparing the total consumption of fish and squids with total prey abundance we can assess an exploitation degree of the forage base and the food capacity. The highest consumption by nekton of food resources in deep-sea regions was recorded in summer (from the third 10-day period of July until the second 10-day period of September). The main consumers were immature salmons which do feeding migrations and squid which abundance increase from early-summer to autumn. Thus, the daily coefficient of grazing pressure in summer were the highest ones—0.0007 in the Commander Basin and 0.0016 in the western Aleutian Basin. In early summer and in autumn, this parameter changed from 0.0003 to 0.0009. In all seasons, the coefficient of grazing pressure was higher in the western part of the Aleutian Basin. The very low values of grazing pressure coefficients imply that food capacity fairly fulfill nekton’s demands. For such groups of zooplankton as amphipods, pteropods, and euphausiida that are not dominant in plankton but are actively consumed by fish and squid, this parameter varied within 0.0001–0.014. The coefficient of use for copepods and sagitts (Sagitta, Chaetognatha), which have a high biomass and high portion in zooplankton community but that are a secondary food in the diet of most mass species, was lower: 0.00005–0.0001 and 0.0001–0.0006, respectively. Copepods and sagitts may consist a considerable reserve for food capacity for fish and squid in the upper epipelagial of deep-sea basins. High abundance of secondary prey and as a consequence high availability may potentially provide alleviation of the grazing pressure on primary prey such as amphipods, euphausiids, and pteropods. In turn, the load on these groups of plankton is also redistributed in autumn when the amphipods increase and euphausiids decrease their abundance. Small-sized nekton also makes an important to the forage base of many fish species. Model estimations showed that the abundance of small-sized nekton in the deep-sea part of the sea may be considerable in summer–autumn: 78–507 (228) mg/m3 (Zavolokin and Glebov, 2009).
Throughout the summer-autumn period, Pacific salmons were the major consumers of zooplankton and nekton resources in the upper epipelagial of deep-sea regions. Due to spatial and temporal separation of different salmon species and size groups within one specie, salmon, even at high densities, did not consume so much food that might influence the functioning of pelagic communities of the Bering Sea and lead to critical situations. This confirms the outputs obtained in previous studies (Naydenko, 2007, 2009, 2010; Shuntiov and Temnykh, 2008a, 2008b, 2011; Zavolokin, 2011, 2014; Shuntov, 2016).
Thus, using the Western Bering Sea deep basins as an example, we consider that the composition, abundance, period of spawning and feeding migrations, spatial and vertical distribution, feeding habits, and plankton-nekton trophic interrelations are not just a result of adaptations and relationships between species but are the mechanisms controlling plasticity of trophic webs, which contribute to stability of ecosystems in turn.
REFERENCES
Aksyutina, Z.M., Elementy matematicheskoi otsenki rezul’tatov nablyudenii v biologicheskikh i rybokhozyaistvennykh issledovaniyakh (Elements of Mathematical Evaluation of the Observation Results in Biological and Fishery Studies), Moscow: Pishchevaya Prom-st’, 1968.
Aydin, K.Y., Lapko, V.V., Radchenko, V.I., and Livingston, P.A., A Comparison of the Eastern Bering and Western Bering Sea Shelf and Slope Ecosystems Through the Use of Mass-Balance Food Web Models, NOAA Technical Memorandum NMFS-AFSC-130, Springfield, VA: U.S. Dep. Commerce, 2002.
Birman, I.B., Morskoi period zhizni i voprosy dinamiki stada tikhookeanskikh lososei (Marine Life Period and Herd dynamics of Pacific Salmons), Moscow: Agropromizdat, 1985.
Bugaev, V.F., Vronskii, B.B., Zavarina, L.O., et al., Ryby reki Kamchatka (chislennost’, promysel, problemy) (Fishes from the Kamchatka River: Abundance, Fishery, and Problems), Petropavlovsk-Kamchatski: Kamchatpress, 2007.
Catalog of Fishes: Genera, Species, References, Version 03/2018, Fricke, R., Eschmeyer, W.N., and van der Laan, R., Eds., 2018. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
Chereshnev, I.A., Volobuev, V.V., Shestakiv, A.V., and Frolov, S.V., Lososevidnye ryby Severo-Vostoka Rossii (Salmon-Like Fishes of the Northeastern Russia), Vladivostok: Dal’nauka, 2002.
Chuchukalo, V.I. Claculation methods of diurnal food diet of fishes, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1996, vol. 119, pp. 289–305.
Chuchukalo, V.I. Pitanie i pishchevye otnosheniya nektona i nektobentosa v dal’nevostochnykh moryakh (Feeding and Trophic Relations of Nekton and Nektobenthos in Far Eastern Seas), Vladivostok: TINRO Tsentr, 2006.
Chuchukalo, V.I., Kuznetsova, N.A., and Napazakov, V.V., Seasonal distribution of euphausiids in the Bering and Okhotsk seas and the adjacent waters of the Pacific Ocean, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1996, vol. 119, pp. 256–281.
Dulepova, E.P., Trophic relations of mass salmon species in the Bering Sea in summer, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1998, vol. 124, pp. 614–623.
Efimkin, A.Ya., Feeding and trophic relations of mass species of nekton in the epipelagial of the Bering Sea, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Vladivostok: TINRO Center, 2006.
Gascuel, D. and Pauly, D., EcoTroph: modeling marine ecosystem functioning and impact of fishing, Ecol. Model., 2009, vol. 220, pp. 2885–2898. https://doi.org/10.1016/j.ecolmodel.2009.07.031
Glebov, I.I., Distribution and migration of the sockeye salmon (Oncorhynchus nerka) from the western part of the Bering Sea in summer–autumn 2002–2006, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2007a, vol. 151, pp. 61–74.
Glebov, I.I., Distribution and migration of the coho salmon (Oncorhynchus kisutch) from the western part of the Bering Sea in summer–autumn 2002–2006, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2007b, vol. 151, pp. 75–82.
Glebov, I.I., Distribution and migration of the chinook salmon (Oncorhynchus tshawytscha) from the western part of the Bering Sea in summer–autumn 2002–2006, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2007c, vol. 151, pp. 83–95.
Glebov, I.I., Savinykh, V.F., and Baitalyuk, A.A., Subtropical migrants in the southwestern part of the Bering Sea, J. Ichthyol., 2010, vol. 50, no. 6, pp. 430–444. https://doi.org/10.1134/S0032945210060020
Gorbatenko, K.M., Feeding of pink salmon juveniles in the epipelagial of the Sea of Okhotsk in winter period, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1996, vol. 119, pp. 234–243.
Karpenko, V.I., Andrievskaya, L.D., and Koval’, M.V., Pitanie i osobennosti rosta tikhookeanskikh lososei v morskikh vodakh (Feeding and Specific Growth of Pacific Salmon in the Seas), Petropavlovsk-Kamchatski: Kamchat. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2013.
Klovach, N.V., Ekologicheskie posledstviya krupnomasshtabnogo razvedeniya kety (Ecological Consequences of Large-Scale Reproduction of Chum Salmon), Moscow: VNIRO, 2003.
Kogan, A.V., Diurnal diet and feeding rhythm of the sabrefish in Tsymlyansk Reservoir, Zool. Zh., 1963, vol. 42, no. 4, pp. 596–601.
Makrofauna pelagiali zapadnoi chasti Beringova morya: tablitsy vstrechaemosti, chislennosti i biomassy, 1982–2009 (Macrofauna of Pelagial of the Western Part of the Bering Sea: Tables of Occurrence, Abundance, and Biomass, 1982–2009), Shuntov, V.P. and Bocharov, L.N., Eds., Vladivostok: TINRO Tsentr, 2012.
Mel’nikov, I.V. and Efimkin, A.Ya., The young of the northern Atka mackerel Pleurogrammus monopterygius in the epipelagic zone over deep-sea areas of the Northern Pacific Ocean, J. Ichthyol., 2003, vol. 43, no. 6, pp. 424–437.
Naumenko, N.I., Abundance dynamics of Pacific herring Clupea pallasii Val. in the western part of the Bering Sea, Issled. Vodn. Biol. Resur. Kamchat. Sev.-Zap. Chasti Tikhogo Okeana, 2010, no. 16, pp. 140–145.
Naydenko, S.V., Role of Pacific salmons in trophic structure of the epipelagial of the western part of the Bering Sea in summer–autumn 2002–2006, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2007, vol. 151, pp. 214–239.
Naydenko, S.V., The role of Pacific salmon in trophic structure of the upper epipelagic layer in the Western Bering Sea during summer-autumn 2002–2006, NPAFC Bull., 2009, no. 5, pp. 231–241.
Naydenko, S.V., The feeding habits of the Pacific salmon, their trophic status and role in the North Pacific marine ecosystem, in Salmon: Biology, Nutrition, and Consumption, Fish, Fishing and Fisheries Series, Lacopo P. and Riemma M., Eds., New York: Nova Science, 2010, pp. 61–100.
Naydenko, S.V., Efimkin, A.Ya., Volkov, A.F., et al., Trophic position of Pacific salmon juveniles in the western Bering Sea epipelagic communities during autumn, NPAFC Tech. Rep., 2007, no. 7, pp. 40–41.
Naydenko, S.V., Efimkin, A.Ya., Lazhentsev, A.E., et al., Selective nutrition of pink salmon (Oncorhynchus gorbuscha) juveniles in Far Eastern seas, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2008, vol. 152, pp. 18–36.
Nekton zapadnoi chasti Beringova morya. Tablitsy chislennosti, biomassy i sootnosheniya vidov (Nekton of the Western Part of the Bering Sea: Tables of Abundance, Biomass, and Species Ratio), Shuntov, V.P. and Bocharov, L.N., Eds., Vladivostok: TINRO Tsentr, 2006.
Nesis, K.N., Gonatid squids in the subarctic north Pacific: ecology, biogeography, niche diversity and role in the ecosystem, Adv. Mar. Biol., 1997, vol. 32, pp. 243–324. https://doi.org/10.1016/S0065-2881(08)60018-8
Nikol’skii, G.V., The pattern of trophic relations between freshwater fishes, in Ocherki po obshchim voprosam ikhtiologii (Essays on the General Problems in Ichthyology), Moscow: Akad. Nauk SSSR, 1953, pp. 261–281.
Radchenko, V.I., Composition, structure and dynamics of nektonic communities of epipelagial of the Bering Sea, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Vladivostok: TINRO Center, 1994.
Radchenko, V.I., Ecopath model as a prospective tool for a comparative study of the functions of the ecosystems of the Okhotsk and Bering seas, Materialy 1-i otkrytoi mezhdunarodnoi konferentsii “Perspektivnye metody otsenki izmenenii geofizicheskikh yavlenii, ekosistem i tekhnologicheskikh protsessov pri izuchenii i osvoenii prirodnykh resursov subarkticheskogo Okhotomor’ya” (Proc. First Open Int. Conf. “Advanced Evaluation Methods of Dynamics of Geophysical Phenomena, Ecosystems, and Technological Processes in the Study and Exploration of Natural Resources of Subarctic Zone of the Sea of Okhotsk”), Yuzhno-Sakhalinsk, 2011, pp. 70–81.
Radchenko, V.I., Modeling of characteristics of the Sea of Okhotsk ecosystem, Tr. VNIRO, 2015, vol. 155, pp. 79–111.
Radchenko, V.I., Volkov, A.F., and Figurkin, A.L., Specific seasonal conditions of planktonic and fish communities of the epipelagial of the Bering Sea, in Ekologiya morei Rossii. Kompleksnye issledovaniya ekosistemy Beringova morya (Ecology of the Russian Seas: complex Studies of the Bering Sea Ecosystem), Moscow: VNIRO, 1995, pp. 326–335.
Romanova, G.P., Nutrition of perch fingerlings in Rybinsk Reservoir, Tr. Biol. Stn. Borok, Akad. Nauk SSSR, 1958, no. 3, pp. 20–25.
Rukovodstvo po izucheniyu pitaniya ryb (Manual for Analysis of Fish Nutrition), Vladivostok: Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1986.
Shebanova, M.A., Chuchukalo, V.I., and Gorbatenko, K.M., Life cycles and somatic production of hyperiid amphipods in the Okhotsk and Bering seas, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2014, vol. 176, pp. 155–176.
Shuntov, V.P., Biologiya dal’nevostochnykh morei Rossii (Biology of Far Eastern Seas of Russia), Vladivostok: TINRO-Tsentr, 2001, vol. 1.
Shuntov, V.P., Biologiya dal’nevostochnykh morei Rossii (Biology of Far Eastern Seas of Russia), Vladivostok: TINRO-Tsentr, 2001, vol. 2.
Shuntov, V.P. and Temnykh, O.S., Long-term dynamics of biota of macroecosystems of the Bring Sea and its environmental factor, Part 2. Modern status of pelagic and bottom communities of the Bering Sea, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2008a, vol. 155, pp. 33–65.
Shuntov, V.P. and Temnykh, O.S., Tikhookeanskie lososi v morskikh i okeanicheskikh sistemakh (Pacific Salmons in Marine and Oceanic Ecosystems), Vladivostok: TINRO Tsentr, 2008a, vol. 1.
Shuntov, V.P. and Temnykh, O.S., Tikhookeanskie lososi v morskikh i okeanicheskikh sistemakh (Pacific Salmons in Marine and Oceanic Ecosystems), Vladivostok: TINRO Tsentr, 2011, vol. 2.
Shuntov, V.P., Volkov, A.F., Matveev, V.I., et al., Productive zone in the Sea of Okhotsk in autumn, Biol. Morya (Vladivostok), 1986, no. 4, pp. 57–65.
Shuntov, V.P., Volkov, A.F., and Efimkin, A.Ya., Composition and modern state of pelagic fish communities of the western part of the Bering Sea, Biol. Morya (Vladivostok), 1988a, no. 2, pp. 56–65.
Shuntov, V.P., Volkov, A.F., and Efimkin, A.Ya., The composition and current state of the Pacific pelagial fish communities in Kamchatka and the Kuril Islands, Biol. Morya (Vladivostok), 1988b, no. 4, pp. 54–62.
Shuntov, V.P., Volkov, A.F., Temnykh, O.S., and Dulepova, E.P., Mintai v ekosistemakh dal’nevostochnykh morei (Walleye Pollock in Ecosystems of Far Eastern Seas), Vladivostok: Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1993.
Shuntov, V.P., Temnykh, O.S., Naydenko, S.V., et al., Substantiation of ecological capacity of the Far Eastern seas and subarctic Pacific for large-scale farming of Pacific salmons, Part 4. Influence of density on food supply for Pacific salmons and their role in food consumption of nekton, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2010, vol. 161, pp. 25–52.
Somov, A.A., The seasonal dynamics of the abundance and species composition of nekton in the upper epipelagic layer of the western Bering Sea, Russ. J. Mar. Biol., 2017, vol. 43, no. 7, pp. 535–554. https://doi.org/10.1134/S1063074017070057
Starovoitov, A.N., The chum salmon (Oncorhynchus keta (Walbaum) in Far Eastern seas: biological characteristic of species. 1. Seasonal distribution and migration of chum salmon in Far Eastern seas and open waters of northwestern Pacific, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2003a, vol. 132, pp. 43–81.
Starovoitov, A.N., The chum salmon (Oncorhynchus keta (Walbaum) in Far Eastern seas: biological characteristic of species. 2. Nutrition and trophic relations of the chum salmon in the epipelagial of Far Eastern seas and adjacent waters of the Pacific Ocean, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2003b, vol. 133, pp. 3–34.
Starovoitov, A.N., Somov, A.A., Emelin, P.O., and Maznikova, O.A., Pacific salmons within the nekton of upper epipelagial of the southwestern part of the Bering Sea in October 2014, in Byulleten’ no. 9 izucheniya tikhookeanskikh lososei na Dal’nem Vostoke (Bulletin No. 9 of the Study of Pacific Salmons of the Far East), Vladivostok: TINRO Tsentr, 2014, pp. 173–184.
Stepanenko, M.A., Interannual variability of spatial differentiation of the Alaska pollock Theragra chalcogramma and Atlantic cod Gadus morhua from the Bering Sea, Vopr. Ikhtiol., 1997, vol. 37, no. 1, pp. 19–26.
Stepanenko, M.A., Resources, interannual variability of abundance, and commercial use of walleye pollock from East Bering Sea population in 80–90’s, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2001, vol. 128, pp. 145–152.
Volkov, A.F., Specific feeding of the pink, chum, and sockeye salmons during anadromous migrations, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1994, vol. 116, pp. 128–136.
Volkov, A.F., Sampling method of zooplankton, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1996, vol. 119, pp. 306–311.
Volkov, A.F., The method of collection and processing of plankton and samples of nekton feeding: step-by-step instruction, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2008, vol. 154, pp. 405–416.
Volkov, A.F., Introduction to walleye pollock trophology, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2015, vol. 183, pp. 166–185.
Volkov, A.F., Gorbatenko, K.M., and Efimkin, A.Ya., Nutrition strategy of walleye pollock, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1990, vol. 111, pp. 123–132.
Volkov, A.F., Efimkin, A.Ya., and Chuchukalo, V.I., Regional features of nutriting of Asian salmons in summer, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1997, vol. 122, pp. 324–341.
Volvenko, I.V., Quantitative evaluation of fish abundance according to trawl surveys, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 1998, vol. 124, pp. 473–500.
Volvenko, I.V., Morphometric characteristics of standards of biostatistical regions for biocenotic studies of fishery zone of Russian Far East, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2003, vol. 132, pp. 27–42.
Volvenko, I.V., Use of new database on the net zooplankton from Far Eastern seas and adjacent waters of Pacific Ocean, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2016, vol. 187, pp. 19–47.
Watanabe, H., Kubodera, T., Moku, M., and Kawaguchi, K., Diel vertical migration of squid in the warm core ring and cold water masses in the transition region of the western North Pacific, Mar. Ecol.: Progr. Ser., 2006, vol. 315, pp. 187–197. https://doi.org/10.3354/meps315187
Zavolokin A.V., Selectivity of nutrition and food availability for Pacific salmons in the Russian zone of the Bering Sea, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Vladivostok: TINRO Center, 2008.
Zavolokin, A.V., Comparative characteristics of food supply of Pacific salmon (Oncorhynchus spp.) in the Bering Sea from 2002 to 2006, J. Ichthyol., 2011, vol. 51, no. 3, pp. 227–219. https://doi.org/10.1134/S0032945211010152
Zavolokin, A.V., Food availability for Pacific salmons during marine and ocean fattening period, Extended Abstract of Doctoral (Biol.) Dissertation, Vladivostok: TINRO Center, 2014.
Zavolokin, A.V. and Glebov, I.I., Low nekton abundance in the Western Bering Sea according to trawl survey data and model estimates, Russ. J. Mar. Biol., 2009, vol. 35, no. 7, pp. 558–573. https://doi.org/10.1134/S1063074009070050
Zavolokin, A.V., Radchenko, V.I., and Kulik, V.V., Dynamics of the trophic structure of the epipelagic community of the western part of the Bering Sea, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2014, vol. 179, pp. 204–219.
Zavolokin, A.V., Radchenko, V.I., and Naydenko, S.V., Changes in the trophic structure of an epipelagic community in the western Bering Sea and western North Pacific Ocean with an emphasis on Pacific salmon (Oncorhynchus spp.), NPAFC Bull., 2016, no. 6, pp. 259–278. https://doi.org/10.23849/npafcb6/259.278
Zheltenkova, M.V., Critical assessment of the modern study methods of the nutrition of fish in natural conditions, Trudy soveshchaniya po metodike izucheniya kormovoi bazy i pitaniya ryb (Proc. Meeting on the Study Methods of Food Resources and Feeding of Fishes), Moscow: Akad. Nauk SSSR, 1955a, pp. 22–39.
Zheltenkova, M.V., Nutrition and use of food resources by bottom fishes in the Sea of Azov, Tr. VNIRO, 1955b, vol. 31, pp. 306–336.
Zheltenkova, M.V., Specific fattening of fishes and rational use of natural reservoir resources, in Trofologiya vodnykh zhivotnykh. Itogi i zadachi (Trophology of Aquatic Animals: Results and Objectives), Moscow: Nauka, 1973, pp. 276–298.
Zuev, M.A., Katugin, O.N., Shevtsov, G.A., and Dakus, A.V., Distribution and differentiation of the boreopacific armhook squid Boreoteuthis borealis (Sasaki, 1923) (Cephalopoda, Gonatidae) in the Sea of Okhotsk and the northwestern part of the Pacific Ocean, Tr. VNIRO, 2007, vol. 147, pp. 266–283.
ACKNOWLEDGEMENTS
We are grateful to all participants of voyages in the Bering Sea and in Pacific waters for collection and primary processing of the material used in the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interests.
Statement of the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by N. Smirnov
Rights and permissions
About this article
Cite this article
Naydenko, S.V., Somov, A.A. Seasonal Trophodynamics of the Upper Epipelagic Nekton Community in the Western Bering Sea. J. Ichthyol. 59, 786–804 (2019). https://doi.org/10.1134/S0032945219050096
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945219050096