Abstract—Cryogenic treatments including shallow and deep cryogenic treatment are supplemental operations designed to improve wear resistance and increase the hardness of a variety of tool and hardening steels. In this research, the effect of cryogenic treatment was investigated on the microstructure and wear behavior (wear behavior in environment temperature and at 550°C) in H11 hot work tool steel. To do so, the samples were austenitized in 1050°C for 60 min and were quenched in oil. Then, the samples were put into dry ice (‒80°C) and liquid nitrogen (–196°C) under shallow and deep cryogenic treatments. Later, the samples were tempered at 550°C for 60 min. Scanning electron microscopy (SEM), optical microscopy (OM), and X-ray diffraction (XRD) analysis were used to analyze the microstructure and the pin on disk method was used to analyze the wear behavior. The results showed that the percentage of retained austenite reaches from 6.5% in quench–temper treatment (QT) to 3% in shallow and to less than 1% in deep cryogenic treatments. Moreover, the tiny carbides are generated (after tempering at 550°C) as a result of deep cryogenic treatment (DCT) and the amount of carbides reaches from 5.5% in QT treatment to 8.2% in DCT treatment. Moreover, in both deep and shallow cryogenic treatments compared to a quench-temper one with the hardness of 4 and 9%, wear resistance at ambient temperature reached 31 and 36% and the wear resistance at high temperature reached 30 and 40%. Additionally, the wear mechanism becomes an adhesive and tribochemical wear in the environment temperature and becomes an abrasive and tribochemical wear in the high temperatures. Conducting cryogenic treatment reduces the amount of adhesive and abrasive wear at the environment and high temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
In recent decades, cryogenic treatment has been introduced to the industry as a heat treatment to improve the micro structure and mechanical properties in a wide variety of materials, especially tool steels, carburized steels, tungsten carbides, composites, and polymers. The cryogenic treatment can be divided into two different types, including Shallow Cryogenic Treatment (SCT) performed at –50 to –80°C and Deep Cryogenic Treatment (DCT) usually performed at temperatures below –120°C [1]. In steel, this treatment is usually performed after the conventional heat treatment and before the tempering process. The purpose of shallow cryogenic treatment is to remove or reduce the retained austenite to increase hardness and thermal stability. In deep cryogenic treatment, two purposes are pursued to not only increase hardness, but also improve wear resistance. These two purposes are:
(1) Removal or reduction of retained austenite.
(2) An increase in volume loss, size reduction, and better distribution of the carbide [2–6].
In a study conducted by Bensely et al. [7] on En353 steel, it was found that shallow and deep cryogenic treatments for 5 and 24 h eliminated complete retained austenite and increased the wear resistance by 85 and 372%, respectively. Meng [8] showed a decrease in the retained austenite equal to 33 and 39% while applying shallow cryogenic treatment on Fe–1.4Cr–1C steel at temperatures of –80 and –196°C, and wear resistance in the deep cryogenic samples has improved with respect to the shallow cryogenic and quench-temper specimens. According to the studies carried out by Koneshloo et al. on H13 hot working steel, the improvement in wear resistance and reduction of retained austenite were reported during two shallow and deep cryogenic treatments [9]. Meng [10] also showed that despite the fact that the percentage of martensite did not change at –50 and –185°C, the study showed that the wear resistance of the sample on which the deep cryogenic treatment was applied experienced some improvement due to the precipitation of η-carbides. The reason behind sediment deposition is network contraction and putting pressure on carbon atoms while leaving the primary locations [10–12]. According to the studies conducted by Huang on steel M2, the improvement in wear resistance in the cryogenic treatment compared to conventional treatments can be attributed to the austenite degradation to martensite and the deposition of alloy carbides. Due to the low amount of retained austenite in this steel, the role of the second factor is more effective. The role of this factor consists in the following: (1) better distribution of alloy carbides; (2) twofold increase in the volume fraction of the carbides in subzero samples compared to the conventional heat treatment. The researchers also believed that the deposition of more carbides in cryogenic treatments will cause a deterioration of the alloys and carbon, which improves the toughness of the M2 steel [12].
H11 tool steel is a hot working type of steel that is widely used in the manufacture of under pressure casting molds, extrusion molding, and extrusion of non-ferrous metals. In this steel, due to the relatively high proportion of alloying elements, there is the likelihood of the presence of retained austenite. Therefore, in the present study, the effect of cryogenic treatments in the shallow cryogenic and deep cryogenic treatments on the microstructure and wear behavior of this steel will be compared with the quench-temper treatment. Moreover, since this steel is a hot working tool, it will be exposed to cold and high temperature wear tests to simulate industrial applications.
2 MATERIALS AND METHODS
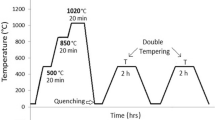
The chemical composition of the H11 steel is shown in Table 1. Preparation of the sample was carried out using the wire cut method and in the form of discs of 50 mm diameter and 5 mm thickness of the original steel specimen. The heat treatment of the specimens consisted of preheating treatments at 650°C for 1 h and austenitizing at 1050°C for 1 h and finally quenching in oil. After that, the samples were divided into three classes. The first part was kept at 550°C, i.e., tempered (QT), and the other two parts in dry ice for 24 h at a temperature of –80°C (SCT) and liquid nitrogen at a temperature of –196°C (DCT), and then they were kept under a tempering treatment at 550°C for 1 h. Hardness tests were performed by Rockwell C, and micro-hardness test of samples was performed using Vickers method with Future FM-700 Hardness testing device (Japan) with 300 g load capacity. To study the samples’ microstructural changes, their surfaces were etched in (a) 100 mL of H2O, 10 g of K3Fe(CN)6 and10 g of NaOH, (b) mixed acid (3 mL of HF, 53 mL of H2NO3, 2 mL of CH3COOH, and 42 mL of H2O). The samples were etched in each of these etchants for 2 s and for 3–4 times. This combination only visualize the carbides and does not affect other phases. The samples surface was then analyzed by optical microscopy. The micrographs were then analyzed via Clemex Vision (version 3.5.025) image analyzing software to calculate the carbide percentage. X-ray diffraction (XRD) (Philips PW1730) with Cu Kα radiation was used to clarify the phases and the retained austenite percentage. The retained austenite percentage was calculated according to ASTM E975-00 standard. The combination of the phase percentage should be equal to 100%. The carbide percentage was evaluated by using the SEM micrographs and the austenite percentage was calculated as follows:
where Vγ and Vc are the retained austenite and carbide percentage, respectively, Iγ and Iα are the integrated intensity per angular diffraction peak (hkl) in the austenite and martensite phases, respectively, and Rγ and Rα are the austenite and martensite constants, respectively. The value of R can be calculated with respect to the (hkl) plane in combination with the polarization, multiplicity, and structure factor of the phases according to the ASTM E975-00 standard [13].
For metallography of the samples, at first they were polished using the usual method, and for microstructure analysis, scanning electron microscopy (SEM, Philips XI30 model, the Netherlands) was used. Moreover, picral etchant solution was used. To perform the cold wear test, a pin of steel bearing with a hardness of 64 HRC was used on the disk. The applied load in the cold wear test was 150 N, the distance was 1000 m, and the test was carried out at air humidity of 30 ± 5% and in the temperature range of 25 ± 5°C at a speed of 0.1 m/s.
The hot wear test was carried out at a high temperature with a carbide tungsten bullet with the pin on ball method. The test was carried out at a load of 25 N and at a speed of 0.1 m/s in the temperature range of 550 ± 10°C and 200 m distance. The wear rate was obtained by using Eq. (2):
where Wr is the wear rate (mm3/N m), Δm is the weight loss (g), ρ is the weight density (g/cm3), F is the load (N), and L is the wear distance (m), in other words, the length of wear path.
3 RESULTS AND DISCUSSION
3.1 Investigating the Microstructure
The X-ray diffraction patterns of the examined samples are shown in Fig. 1. According to the images, the percentage of retained austenite in deep cryogenic treatment is reduced compared to shallow cryogenic and conventional treatments. Table 2 shows the amount of retained austenite. The amount of retained austenite in the QT samples reached from 6.5 to 3% in SCT and less than 1% in the DCT samples. Reduction of the amount of retained austenite has also been proved due to the cryogenic treatment on other steels [7, 9, 14, 15]. The microstructure of samples has been analyzed using OM and SEM (modes of microscopy) in Figs. 2–4. Figure 2 shows the image of OM-studied H11 steel after etching in (a) 100 mL of H2O, 10 g of K3Fe(CN)6, and10 g of NaOH, (b) mixed acid (3 mL of HF, 53 mL of H2NO3, 2 mL of CH3COOH, and 42 mL of H2O) solution. The solution helps revealing the carbides. The percentage of carbides using Image Analyses Software indicates that the volume fraction of carbides reaches from 5.5% in QT sample to 6.4% in SCT sample and to 8.2% in DCT sample. Thus, based on Fig. 2, tiny carbides are precipitated as a result of cryogenic treatment so that the amount of carbides is increased. This takes place with more intensity in the deep cryogenic treatment. Figures 3 and 4 include the OM and SEM images of the structure of samples. Becoming tiny and increased carbide content are clear in the images. The deposition of fine carbides during the deep cryogenic treatment after tempering is due to the fact that the structure is under intense stress because of severe contraction, which is the result of the difference in the coefficient of expansion of austenite and martensite. Therefore, this high stress level causes the driving force for jumping of carbon atoms to the adjacent defects. New defects are a consequence of the difference in the expansion coefficients of austenite and martensite at the boundary of the phases. These two factors serve as new preferences for the creation of carbide nuclei during the tempering treatment [16]. In a study carried out by Amini et al. performed on 80CrMo125 steel, a 2% increase in volume fraction of the carbide was reported due to deep cryogenic treatment compared to the conventional treatment. Moreover, they reported that the distribution of the carbides has been improved due to the cryogenic treatment [1].
In another study conducted by the same researchers on 1.2080 steel, an increase in volume fraction and a better distribution of carbides as well as the formation of nano-metric carbides during deep cryogenic treatments were reported [1, 11, 16, 17]. Because the penetration rate of carbon atoms is very low at ‒196°C, these atoms cannot be positioned around very hard crystalline defects and deposition of fine carbides is impossible, but in the heating of the samples from below zero to ambient temperature, the penetration rate of carbon has increased. Moreover, carbon atoms are diffused by martensite crystalline network, and their very low penetration induces the formation of fine clusters around the crystalline defects, and then leads to the formation of very fine carbides. The deposition process in deep cryogenic treatments which includes martensitic decomposition and the formation of fine carbides is very similar to that of the tempering process in a tempering process, with the exception that due to lower temperatures, carbide sediments are smaller and more appropriately distributed.
3.2 Investigating the Hardness and Wear Behavior of the Sample
According to Table 3, the hardness increases 4 and 9% respectively in result of shallow and deep cryogenic treatments in comparison with the quenched-tempered treatment. This process happens because of the reduction in retained austenite, precipitation of tiny carbides, increase in the carbide content, and their better distribution. Since the effect of deep cryogenic treatment is more intense in reducing the retained austenite and precipitating the tiny carbides, a more increase is observed in the hardness amount because of deep cryogenic treatment. In a research on AISI 440C steel [18], the increase in the Rockwell hardness in the shallow and deep cryogenic treatment samples was reported to be 4 and 7%, respectively. In this study, the increase in the percentage of retained austenite transformation to martensite has been the cause of the increase in hardness. In another study carried out by Akhbarizadeh et al. on D6 steel, the increase in the Rockwell hardness of the shallow and deep cryogenic samples was 1.6 and 3.2%, respectively, compared to the conventional heat treatment samples. They reported that this hardness was associated with the retained austenite transformation to martensite as well as deposition of the secondary carbides in deep cryogenic treatment [14].
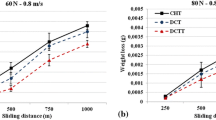
Figures 5, 6, and Table 4 show the results of the wear tests at ambient temperature and higher temperatures. As can be clearly observed, shallow and deep cryogenic treatments have increased the wear resistance by 31 and 36% at ambient temperature and 30 and 40% at 550°C compared to the quench-tempered sample. The reason for the improvement of wear resistance, according to the obtained results, can be dedicated to the reduction of retained austenite in the shallow cryogenic samples and to the reduction of retained austenite in the deep cryogenic samples along with an increase in the volume fraction, fineness, and better distribution of carbides compared to the quench-tempered sample. According to Fig. 4, as the wear distance increases, the wear rate decreases because of work hardening [19].
Therefore, in addition to reduction or removal of retained austenite as the soft phase in the cryogenic treatment, the precipitation of tiny carbides causes the depletion of steel matrix of carbon and alloying elements. So, the steel matrix possesses higher toughness, and as a result the wear resistance is improved because of matrix’s higher toughness, precipitation of tiny carbides and reduction or removal of detained austenite [10, 12, 20]. In a study on En353 steel, an increase in the wear resistance in shallow and deep cryogenic treatments was 85 and 372%, respectively, which is due to the removal of austenite, deposition, and better distribution of carbides in the deep cryogenic treatments [7].
To determine the wear mechanism, the wear surface of the samples was investigated by SEM (microscopy). The images shown in Figs. 7 and 8 are related to wear at ambient temperature and high temperatures. According to Fig. 7, the dominant wear mechanism is the adhesive wear at the environment temperature. This wear is generated as a result of adhesive joints between the worn-out surfaces as they contact each other. As the joint break occurs in the interface, no wear occurs; but as the break happens in one of these pieces, the wear happens. The closer hardness of surfaces, the more probability of occurrence in this type of wear. In fact, it can be said that frictional heat between the pieces during wear results in adhesive joints, which finally eventuates in generation of removal effects on the surface. According to Fig. 7a, a significant part of surface includes the adhesive patches which are multi-layered in some patches. Therefore, severe adhesive wear occurred in QT sample. The adhesive wear has been reduced by applying the cryogenic treatment (Figs. 7b, 7c).
By conducting the wear test at high temperature with a carbide tungsten bullet, due to the reduction in hardness surface, wear mechanism changes from the adhesive wear to the abrasive wear (Fig. 8). By softening the material surface at higher temperatures, more plastic deformation occurs. Reducing the hardness as the result of temperature increase causes that the inconsistencies in harder surface are injected into the softer surface (pin on disk) and generate scratches in parallel with the softer surface. This wear mechanism primarily leads to material transport from the scratch to the edges (Fig. 8). As it is clear in Fig. 8a, more plastic deformation occurs in the edges in the QT sample because of lower hardness so that micro-ploughing mechanism occurs. In the cryogenic sample due to the higher hardness and higher resistance to the hardness reduction, the resistance against plastic deformation is higher and micro-cutting happens (Fig. 8c). As it is seen in Figs. 9 and 10, oxidation occurs in the wear test due to the friction and high temperature, so that the tribochemical wear occurs. This effect becomes more severe in the sample being exposed to the high temperature under wear test.
A study done by Amini et al. on DIN 1.7131 steel showed a better distribution of carbides and an increase in their volume fraction, a reduction in adhesive wear as well as the removal of retained austenite, which has increased hardness [21]. Adhesive wear is also directly proportional to the applied force and has a reverse proportion to the hardness [22].
Therefore, it can be concluded that in the samples under the cryogenic treatment, the hardness increases due to the reduction of retained austenite, the precipitation of tiny carbides, the increase in the volume fraction, and better distribution of carbides, leading to the increase of the wear resistance. Figure 11 shows the images of the wear products after wearing test. For more studies the wear products were also examined by SEM (microscopy). The results show that the wear derbies of the DCT sample are smaller in size compared with the QT ones. This behavior is a consequence of the harder surface of the DCT samples compared to the conventionally treated ones. This has also been proved by other scientists [14].
4 CONCLUSIONS
(1) Cryogenic treatment leads to the reduction in the amount of the retained austenite. The amount of retained austenite in the treatment of the quench-temper reached from 6.5 to 3% in shallow cryogenic treatments and less than 1% in deep cryogenic treatment.
(2) As a result of cryogenic treatment, the tiny carbides are precipitated and volume fraction of carbides increases. This effect is higher in deep cryogenic treatment in contrast to shallow cryogenic treatment. Related to the steel studied, the percentage of carbides reaches from 5.5% in QT sample to 6.4 and 8.2% in the SCT and DCT samples respectively. Therefore, the deep cryogenic treatment has created a greater driving force for the formation of very fine carbides.
(3) Due to the shallow cryogenic treatment, hardness and wear resistance at an ambient temperature increased 4 and 31%, and wear resistance at a high temperature—of 30%; and as a result of deep cryogenic treatment, these values were 9, 36, and 40%, respectively, compared to the quench-tempered sample. This is due to a decrease in the amount of retained austenite and to the deposition of fine carbides in cryogenic treatments compared to the quench-temper treatment.
(4) The dominant wear mechanism is the adhesive and tribochemical wear at the environment temperature. The adhesive wear is severe in the QT sample, but the adhesive wear is weak in the cryogenic samples. The reason lays in the increase in hardness in the cryogenic treatment. The wear mechanism is abrasive and tribochemical in the samples that are exposed to wear test at the high temperatures with a carbide tungsten bullet, due to the hardness reduction. In the QT sample due to low hardness, more plastic deformation in scratches (micro-ploughing sub-mechanism) is observed in the edges. In addition, in SCT and DCT samples due to higher hardness, a pattern of scratches without plastic deformation (micro-cutting sub-mechanism) is observed. Moreover, the collected wear derbies of the cryogenically treated samples were more brittle and smaller.
REFERENCES
K. Amini, S. Nategh, A. Shafyei, and A. Rezaeian, “Effect of deep cryogenic treatment on the properties of 80CrMo12.5 tool steel,” Inter. J. Miner., Metall. Mater. 19, 30–37 (2012).
K. Amini, S. Nategh, A. Shafiey, and M. A. Soltany, “To study the effect of cryogenic heat treatment on the hardness and the amount of residual austenite in 1/2304 steel,” Metal. 13, 1–7 (2008).
F. Cajner, V. Leskovšek, D. Landek, and H. Cajner, “Effect of deep-cryogenic treatment on high speed steel properties,” Mater. Manufact. Processes 24, 743–746 (2009).
D. Das, A. Dutta, and K. Ray, “Influence of varied cryotreatment on the wear behavior of AISI D2 steel,” Wear 266, 297–309 (2009).
D. Das, A. K. Dutta, and K. K. Ray, “Sub-zero treatments of AISI D2 steel: Part I. Microstructure and hardness,” Mater. Sci. Eng., A 527, 2182–2193 (2010).
D. Das, K. Ray, and A. Dutta, “Influence of temperature of sub-zero treatments on the wear behaviour of die steel,” Wear 267, 1361–1370 (2009).
A. Bensely, A. Prabhakaran, D. M. Lal, and G. Nagarajan, “Enhancing the wear resistance of case carburized steel (En 353) by cryogenic treatment,” Cryogenics 45, 747–754 (2005).
F. Meng, K. Tagashira, and H. Sohma, “Wear resistance and microstructure of cryogenic treated Fe–1.4 Cr–1C bearing steel,” Scr. Metall. Mater. 31, 865–868 (1994).
M. Koneshlou, K. M. Asl, and F. Khomamizadeh, “Effect of cryogenic treatment on microstructure, mechanical and wear behaviors of AISI H13 hot work tool steel,” Cryogenics 51, 55–61 (2011).
F. Meng, K. Tagashira, R. Azuma, and H. Sohma, “Role of eta-carbide precipitations in the wear resistance improvements of Fe–12Cr–Mo–V–1.4 C tool steel by cryogenic treatment,” ISIJ Int. 34, 205–210 (1994).
K. Amini, A. Akhbarizadeh, and S. Javadpour, “Effect of deep cryogenic treatment on the formation of nano-sized carbides and the wear behavior of D2 tool steel,” Intern. J. Miner., Metall. Mater. 19, 795–799 (2012).
J. Huang, Y. Zhu, X. Liao, I. Beyerlein, M. Bourke, T. Mitchell, “Microstructure of cryogenic treated M2 tool steel,” Mater. Sci. Eng., A 339, 241–244 (2003).
A. Standard, “E975-03: Standard practice for X-ray determination of retained austenite in steel with near random crystallographic orientation,” ASTM, West Conshohocken, PA (2008).
A. Akhbarizadeh, A. Shafyei, and M. Golozar, “Effects of cryogenic treatment on wear behavior of D6 tool steel,” Mater. Design 30, 3259–3264 (2009).
D. Senthilkumar, I. Rajendran, M. Pellizzari, and J. Siiriainen, “Influence of shallow and deep cryogenic treatment on the residual state of stress of 4140 Steel,” J. Mater. Proces. Techn. 211, 396–401 (2011).
A. Akhbarizadeh, S. Javadpour, K. Amini, and A. H. Yaghtin, “Investigating the effect of ball milling during the deep cryogenic heat treatment of the 1.2080 tool steel,” Vacuum 90, 70–74 (2013).
K. Amini, A. Akhbarizadeh, and S. Javadpour, “Cryogenic heat treatment of the ferrous materials—A review of the current state,” Metal. Res. Techn. 113, 611 (2016).
A. Idayan, A. Gnanavelbabu, and K. Rajkumar, “Influence of deep cryogenic treatment on the mechanical properties of AISI 440C bearing steel,” Procedia Eng. 97, 1683–1691 (2014).
G. Fontalvo, R. Humer, C. Mitterer, K. Sammt, I. Schemmel, “Microstructural aspects determining the adhesive wear of tool steels,” Wear 260, 1028–1034 (2006).
H. Paydar, K. Amini, and A. Akhbarizadeh, “Investigating the effect of deep cryogenic heat treatment on the wear behavior of 100Cr6 alloy steel,” Kovove Mater.-Met. Mater. 52, 163–169 (2014).
K. Amini, A. Araghi, and A. Akhbarizadeh, “Effect of deep cryogenic heat treatment on the wear behavior of carburized DIN 1.7131 grade steel,” Acta Metal. Sinica (English Letters) 28, 348–353 (2015).
B. Bhushan, Introduction to Tribology (Wiley, New York, 2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jalil Soleimany, Ghayour, H., Amini, K. et al. The Effect of Deep Cryogenic Treatment on Microstructure and Wear Behavior of H11 Tool Steel. Phys. Metals Metallogr. 120, 888–897 (2019). https://doi.org/10.1134/S0031918X19090035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031918X19090035