Abstract—Changes in the activity of antioxidant systems in Escherichia coli during phosphate starvation have been studied. It is shown that starvation was accompanied by a decrease in the intensity of respiration, an increase in the rate of superoxide production, and a decrease in the level of ATP. Simultaneously, there was a decrease in H2O2 in the medium and a significant increase in the expression of the katG and katE genes which encode the HPI and HPII catalases, respectively. At the same time, there was no drop in the membrane potential, which may indicate the retention of normal membrane activity in starving cells. It has been shown for the first time that the transition of E. coli to phosphate starvation is accompanied by significant changes in the status of glutathione. The most important of these are associated with a decrease in the level of reduced glutathione in the medium (GSHout) and with a simultaneous increase in its content in the cytoplasm (GSHin), as well as a shift in the GSHin to oxidized glutathione form (GSSGin) ratio towards reductive values, and GSHout/GSSGout towards oxidative values. Among the mutants used in the work, the gor trxB double mutant, which is deficient in the synthesis of glutathione reductase and thioredoxin reductase, showed the most pronounced distinctive features. Compared to the parental strain, this mutant showed a multiple higher expression of katG::lacZ, the highest level of oxidized intra- and extracellular glutathione, and, accordingly, the lowest GSH/GSSG ratio in both compartments. In general, the data we obtained indicate that during phosphate starvation the interaction of the glutathione redox-system and regulons that control protection against reactive oxygen species creates conditions that allow maintaining the concentration of ROS below the toxic level. As a result, phosphate-starved E. coli cells can maintain high viability for a long period of time, which allows them to quickly resume growth after the addition of phosphate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In their natural habitat, bacteria face various stresses, including those caused by the depletion of the main substrates. Most stresses are accompanied by inhibition of growth, so cells have universal mechanisms to deal with the negative consequences of a sharp slowdown in metabolic processes. These consequences may include increased production of reactive oxygen species (ROS) such as the superoxide anion (\({\text{O}}_{2}^{{\centerdot - }}\)), hydrogen peroxide (H2O2) and the hydroxyl radical (HO•), which can damage DNA, proteins, and cell membranes. To avoid the damaging effects of possible oxidative stress, cells tend to maintain ROS at a safe level. Bacteria have a set of enzymes that scavenge ROS and restore damaged macromolecules, as well as sensors and signaling pathways that perceive changes in the redox state and activate the mechanisms of its homeostasis [1].
Escherichia coli contains three superoxide dismutases (SODs): cytoplasmic Mn-SODs (sodA) and Fe-SOD (sodB) and periplasmic Cu-Zn-SOD (sodC). The main enzymes that remove H2O2 in vivo, include the alkyl hydroperoxide reductase Ahp (ahpCF) and catalases HPI (katG) and HPII (katE), whose gene expression is under the control of the transcriptional regulators OxyR and RpoS [2].
In addition, low-molecular-weight thiols are involved in maintaining intracellular redox homeostasis. The cytosol of E. coli contains two main thiol-based redox systems, the functions of which overlap significantly: the glutathione-glutaredoxin system and the thioredoxin system [3, 4]. Glutathione is the main thiol redox buffer. In the cytoplasm, glutathione is predominantly in a reduced state and its intracellular concentration is approximately 5 mM. Oxidized glutathione (GSSG) is reduced by glutathione reductase (GOR), which is encoded by the gor gene and is a part of the OxyR regulon [3]. During exponential growth under aerobic conditions, glutathione can accumulate in the medium at the micromolar level and undergo continuous transmembrane circulation [5].
The thioredoxin system of E. coli consists of two thioredoxins (Trx1 and Trx2) encoded by trxA and trxC, and thioredoxin reductase (TrxR), encoded by trxB [3, 4, 6]. E. coli also contains three glutaredoxins (Grx1, Grx2, and Grx3) encoded by grxA, grxB, and grxC respectively, and two glutaredoxin-like proteins Grx4 (grxD) and NrdH (nrdH). Oxidized glutaredoxins, except for Grx4 and NrdH, are nonenzymatically reduced by glutathione [4, 6].
One of the stressful situations that can potentially be accompanied by disruption of redox homeostasis and ROS production is phosphate starvation (Pi). Phosphate is required by the cells of all living organisms for structural and energy purposes. Under natural conditions, the content of phosphate can vary in a significant range, up to its complete absence. When there is no phosphate in the medium, E. coli activates an adaptive response that includes the synthesis of several dozen proteins, some of which are included in the Pho regulon, which is a global regulatory network that interacts with the biosynthesis of polyphosphates, RpoS, and ppGpp [7, 8]. Proteomic analysis showed that the overall response of E. coli to phosphate starvation can include up to 400 genes, which make up almost 10% of the genome of this bacterium [9].
Previous evidence has been presented that E. coli growing on MOPS medium with glucose and six amino acids, maintain an active metabolism for about 3 days of phosphate starvation. Phosphate-starved cells utilized not only glucose, but also amino acids present in the nutrient medium, producing metabolites such as putrescine and spermidine, which are involved in the protection of nucleic acids from oxidative damage [10–12]. Based on experiments with mutants, it was suggested that the RpoS and LexA regulons, as well as the H-NS protein, are important for maintaining the viability of bacteria [10]. It is known that the expression of katG and katE increases with entry of E. coli into the stationary phase [2]. Considering that phosphate starvation causes bacterial growth arrest and transition to the stationary phase, it would be logical to expect the induction of KatG and KatE in such cells. However, VanBogelen et al. [9] did not find the induction of these catalases in response to starvation of E. coli for phosphate. Moreau et al. [11] found that a deficiency in the synthesis of alkyl hydroperoxide reductase (Ahp) and catalase HPI (KatG) significantly reduced the survival of phosphate-starved cells; however, gene inactivation of oxyR and rpoS had only a small effect on survival. The authors judged the production of ROS indirectly by measuring the concentrations of compounds that react with thiobarbituric acid. This method is often criticized for its lack of specificity.
In experiments with other bacteria, Sinorhizobium meliloti, it was shown that phosphate starvation stimulated H2O2-inducible catalase (katA) gene expression in cells. It was found that for gene transcription of katA a PhoB regulator was needed, and initiation occurred on a promoter other than the OxyR-dependent one that activates transcription of katA in response to adding H2O2 [13]. The authors observed a similar effect of starving for phosphate in Agrobacterium tumefaciens and Pseudomonas aeruginosa. Based on the fact that phoB-mutants were more sensitive to H2O2 than mutants katA, Yuan et al. assumed that in addition to katA, phosphate starvation induces other PhoB-regulated genes that play an important role in protecting cells from H2O2 [13].
In general, the previously obtained data leave open the question of ROS production and the role of oxidative stress in phosphate-starving bacteria.
We have recently shown that during amino-acid starvation of E. coli growing under aerobic conditions glutathione plays an important role in response to stress [14]. Bacteria maintain a low level of intracellular free cysteine due to its high reducing activity towards Fe3+ ions, which contributes to the Fenton reaction [15, 16]. Unlike cysteine, GSH is a weak iron reducer in the highest oxidation state [15], so the incorporation of cysteine into glutathione serves as an effective mechanism for maintaining cysteine homeostasis with an increase in its intracellular concentration, which is observed when protein synthesis is stopped [14]. Reducing the excess of cysteine in cells is also achieved by its export to the medium and desulfurization with the formation of H2S [14, 16, 17]. We have previously shown that amino acid starvation stimulates an increase in the intra- and extracellular pool of glutathione, as well as a change in the ratio of reduced/oxidized glutathione in the cytoplasm (GSHin/GSSGin) [14]. It is known that the redox status of glutathione and other thiol redox systems are closely interrelated both with each other and with the expression of enzymes that neutralize ROS [3].
Based on the data, it can be concluded that understanding the dynamics of changes in the redox status of glutathione will expand our knowledge of the mechanisms of bacterial response to stress induced by starvation. In this work, we studied changes in the activity of antioxidant systems during phosphate starvation of E. coli. We evaluated such indicators as the redox status of glutathione, the production of H2O2, and expression of antioxidant enzymes.
EXPERIMENTAL
Materials and reagents. We used reagents from Sigma Chemical Co.: 3-[N-morpholine]propanesulfonic acid (MOPS), 2-nitrophenyl-β-D-galactopyranoside, deoxycholate, mercaptoethanol, horseradish peroxidase (HRP), Amplex Red, DiBAC4(3), ferricytochrome C, superoxide dismutase, 5,5'-dithiobis(2-nitrobenzoic acid, glutathione reductase; as well as agar (Fluka, United States), LB medium (Amresco, United States), dimethyl sulfoxide (DMSO) (Scharlau, Spain), and ethylenediaminetetraacetic acid (EDTA) (BioFrox, Germany); the remaining reagents were of analytical grade from Russian manufacturers.
Bacterial strains and growth conditions. The Escherichia coli BW25113 parent strain (wt) and single mutants: gshA, gor, trxA and trxB, were from the Keio collection [18] (Table 1). Double mutants: gshA trxA and gor trxB, were constructed by transduction with P1 phage using appropriate Keio strains. The parent strain and mutants listed above, carrying katG::lacZ [19], katE::lacZ [20], sulA(sfiA)::lacZ [21] and iucC::lacZ [22] transcriptional gene fusions were created by methods of transformation and transduction.
Cells were grown in MOPS medium supplemented with 8.5 mM glucose and 2 mM KH2PO4 (or 2 mM NaH2PO4 when using a medium with a low content of K+) [23]. Overnight cultures were centrifuged and diluted in 50 ml of fresh medium to an optical density of 0.1 at 600 nm (OD600) and then grown to OD600 0.6 at 37°C in 250 mL flasks, with shaking at 150 rpm. After centrifugation, cells were diluted to OD600 0.25 in 100 ml of prewarmed fresh medium with phosphate (control) or without phosphate (Pi-starvation) and grown from 2 to 48 h as above. The specific growth rate (µ) was calculated using the equation µ = ΔlnOD600/Δt, where t is time (h).
Measurement of oxygen (dO2) and potassium (K+). Dissolved oxygen monitoring (dO2) was performed in real time directly in flasks using a Clark InPro 6800 oxygen electrode (Mettler Toledo, Switzerland). Changes in the level of extracellular potassium were recorded using an ELIS-121K K+-selective electrode (IT, Russia) directly in flasks in a medium with a low concentration of K+ (0.2 mM), where E. coli cells were added prepared as above. Synchronous processing of all primary data from the sensors was carried out using the RS-232 and Modbus protocols of the Advantech OPC Server v3.0 software package (https://advantech-modbus-opc-server.soft-ware.informer.com/3.0/).
Determination of ATP concentration and the membrane potential. The ATP concentration was measured by the luciferin-luciferase method using the ATP Determination Kit (Molecular Probes, United States). For ATP extraction, 50 µL of the cell suspension was mixed with 450 µl of DMSO. The extraction was carried out for 5 min and the ATP concentration was determined according to the manufacturer’s protocol.
Membrane potential changes were assessed using ∆ψ-sensitive fluorescent dye DiBAC4(3) [24, 25]. Cell samples treated with protonophore carbonyl cyanide-m-chlorophenylhydrazone (20 μM) were used as a positive control. Fluorescent cells were counted using a Leica DM2000 fluorescence microscope (Leica, Germany) as described previously [25]. The total number of cells was counted in transmitted light. Approximately 1000 cells were counted for each sample. All experiments were carried out 3–6 times independently of each other.
Measurement of extracellular superoxide and H2O2. Extracellular superoxide was determined by using it ability to restore ferricytochrome c [26]. To determine H2O2, the cells were grown as described above. At certain intervals, aliquots of the culture with a volume of 2 mL were taken, passed through membrane filters, and the concentration of H2O2 in filtrates was determined using the Amplex Red–Horseradish peroxidase system [27] using a Shimadzu RF-1501 spectrofluorimeter (Shimadzu, Japan) (λex 563 nm and λem 587 nm). The H2O2 concentration in samples was calculated from the calibration curve.
Measuring glutathione and gene expression. Extracellular and intracellular glutathione was determined spectrophotometrically by the cyclic method with 5,5'-dithiobis(2-nitrobenzoic acid) and glutathione reductase [28], modified as described previously [5]. To determine extracellular glutathione, samples of cell suspensions were passed through membrane filters (0.45 μm). One part of the filtrate was analyzed for total glutathione and the other for GSSG content. To determine intracellular glutathione, 10 ml of culture was centrifuged at 8000 g within 5 min, the precipitate was resuspended in 5 ml of a cold aqueous solution of 20 mM EDTA and disrupted by ultrasound at 0°C. Protein precipitation and determination of GSH and GSSG were performed as described previously [5]. Calibration curves were built from known concentrations of GSH and GSSG, which were processed as samples of cell suspensions. The glutathione concentration was expressed as the ratio of the value obtained from the calibration curves to the OD600 at the time of sampling.
Changes in the expression of the analyzed genes were assessed by determining the activity of β-galactosidase [29] of E. coli strains carrying the corresponding gene fusions.
Determination of colony forming units (CFU). To calculate CFU, serial dilutions were prepared (from 10−1 to 10−8) of each sample in 0.9% sterile NaCl. One milliliter of diluted culture (usually 10‒6 and 10‒7 to obtain single colonies) was mixed with 3 ml of molten soft LB-agar (0.8%) at 42°C and poured onto Petri plates with solid LB-agar (1.5%). The number of colonies on the plates was counted after 24 h of incubation at 37°C.
Statistical analysis. Each result is presented as an average value (at least three independent experiments) ± standard error of the mean. For analysis we used the Student’s t-test. Values of p < 0.05 were considered statistically significant. The results were analyzed using the Statistica 8.0.360 program (Statsoft Inc. 2007).
RESULTS
The effect of phosphate starvation on the growth and energy parameters of E. coli
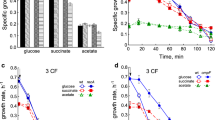
In the absence of phosphate limitation, the maximum specific growth rate (µmax) of the exponentially growing E. coli parent strain (wt) and gshA, gor, trxA, trxB, gshA trxA and gor trxB mutants was 0.69 ± 0.02, 0.71 ± 0.01, 0.65 ± 0.01, 0.54 ± 0.01, 0.63 ± 0.01, 0.63 ± 0.01, and 0.57 ± 0.01 h−1 respectively (Fig. 1a). When the density reached ~1 p.u. at a wavelength of 600 nm, the growth rate gradually decreased in all strains due to a drop in oxygen concentration and accumulation of acidic metabolic products.
After the bacteria were transferred from the growing culture to the medium without phosphate, the growth rate rapidly decreased, reaching a value of about 0.25 h−1 in 20–40 min, after which in all strains (including the parent) a phase of a slower decrease in the growth rate was observed (Fig. 1b). The growth observed in the first phase appeared to be associated with consumption of trace amounts of Pi that entered the medium along with centrifuged cells. After 24 h, growth stopped in all strains. The addition of phosphate resulted in regrowth at the rate seen in the growing culture (data not provided). Growth retardation in cultures growing under conditions of phosphate starvation was also recorded by a decrease in CFU when compared with a culture consuming phosphate (Figs. 1c, 1d). During the first 2 h of incubation of the parent strain oin a medium with phosphate, the number of CFU increased by 4.5 times, while in the starving culture this indicator increased by 2.9, and in the mutants, by an average of 2.1 times. The smallest increase in CFU (by 1.5 times) was observed for the trxA mutant, which is defective in the synthesis of thioredoxin-1.
The parent strain and gshA, gor, trxA, trxB, gshA trxA, and gor trxB mutants, exponentially growing on a medium with phosphate contained 3.07 ± 0.08, 2.85 ± 0.06, 3.13 ± 0.1, 2.34 ± 0.11, 2.80 ± 0.1, 2.84 ± 0.06, and 2.3 ± 0.06 μM ATP/OD600 respectively (zero time in Fig. 2a). When phosphate was depleted, synchronously with a decrease in the growth rate, the ATP level decreased in all strains by an average of 2.3 times after 1 h and by 5.3 times after 24 h (Fig. 2a).
Changes in physiological parameters of E. coli during phosphate starvation. (a) ATP level; (b) is the percentage of cells that have lost their membrane potential (stained with the fluorescent dye DiBAC4(3)); (c) is the content of dissolved oxygen (dO2) and extracellular potassium (K+) in the culture of E. coli BW25113 (wt), 0.5 mV corresponds to 8 µM K+; (d) the extracellular concentration of H2O2 in E. coli BW25113(wt); (e) the accumulation of H2O2 in the culture medium of mutants for thiol redox systems; (f) the rate of accumulation of H2O2 in the medium of the studied strains for 2 h of growth or starvation (nmol O\({\text{D}}_{{600}}^{{ - 1}}\) min–1). *R < 0.05 between the values of the parameters obtained for the mutants relative to the parental strain (b, e) or between values in growing and starving cultures for each strain (f).
In all strains, including the parental one, during growth in the presence of phosphate, the proportion of cells stained with the fluorescent dye DiBAC4(3), varied from 2.0 to 3.2% and changed little during growth (zero time in Fig. 2b). Tteatment with the protonophore carbonyl treatment with carbonyl cyanide-m-chlorophenylhydrazone (20 μM CCCP) increased the number of cells permeable to DiBAC4(3), up to 54% within 20 min (not shown). During 48 h of phosphate starvation, no increase in the number of stained cells was observed in all strains, which indicates that starving cells retained their membrane potential.
A decrease in the growth rate at the initial phase of incubation in a culture medium without phosphate was accompanied by a drop in the oxygen level (Fig. 2c). We hypothesize that this may be due to aerobic glucose metabolism. The falling dO2 rate for the parent strain at this phase was 4.3% O\({\text{D}}_{{600}}^{{ - 1}}\) min−1 compared to 6.3% O\({\text{D}}_{{600}}^{{ - 1}}\) min-1 for the same period after the transfer of bacteria to the medium with phosphate. In all strains, including the parental one, after 10–20 min from the start of incubation, phosphate depletion led to a sharp increase in dO2 and the level of K+ in the medium, which indicates a decrease in oxygen consumption and the release of part of the K+ from cells (Fig. 2c). Then, the slow consumption of oxygen and K+ resumed, corresponding to the growth of bacteria at a low rate. The rate of decrease of dO2 at this phase (40–60 min after transfer to medium without phosphate) was 0.27% O\({\text{D}}_{{600}}^{{ - 1}}\) min−1, which was almost 5 times lower than the corresponding rate in a medium with phosphate (1.28% O\({\text{D}}_{{600}}^{{ - 1}}\) min−1).
The Effect of Phosphate Starvation on Oxidant Production and Antioxidant Gene Expression in E. coli
The growth of bacteria in the presence of phosphate was accompanied by the accumulation of H2O2 in the environment. In a starving culture, the concentration of extracellular hydrogen peroxide was kept at a low level; with the addition of phosphate, the resumption of growth was accompanied by a rapid increase in H2O2 in the medium (Fig. 2g). In the presence of phosphate, for all mutants (except gor) the extracellular concentration of H2O2 was lower than that of the parent strain. The double mutant gshA trxA had the lowest H2O2 level and was 35% of the parental level. For the gor mutant, which is deficient in glutathione reductase, the extracellular concentration of H2O2 was 12% higher than that of the parental strain (Fig. 2e). The accumulation rate H2O2 in the medium, calculated as nmol O\({\text{D}}_{{600}}^{{ - 1}}\) min–1, was minimal in gshA and gshA trxA mutants. In other cases, there was no significant difference from the parent (Fig. 2f). At 1 h after the start of fasting the level of H2O2 in all strains was on average 44% lower than in the growing culture. The degree of reduction was generally proportional to the content of H2O2 before starvation, with the exception of the gor trxB double mutant, whose H2O2 level decreased by 29% from the original. The accumulation rate of H2O2 in the starving culture was also lower than in the growing one in all strains, except gshA (Fig. 2f). Further observation revealed that after 24 h of fasting, the extracellular level of H2O2 in gshA and trxB mutants was restored to values close to those in the growing culture, and in the parental strain and gor and trxA mutants it slightly increased, but did not reach the values in the growing culture. We note the sharp increase (more than 2 times) in the production of H2O2 in double mutants (Fig. 2e).
In contrast to H2O2, the production of superoxide during phosphate starvation not only did not decrease, but even increased by 2–3 times in comparison with the culture that was not limited in phosphate (Fig. 3a). This effect was equally observed both in the parental strain and in the gshA and gshA trxA mutants. An increase in superoxide production corresponded to a slowdown in respiration during starvation (Fig. 2c). The addition of phosphate led to a decrease in superoxide formation to the level observed in the culture with phosphate (Fig. 3a).
Superoxide production and antioxidant gene expression in growing and phosphate-starved E. coli. (a) Production of superoxide in strain BW25113 (wt); (b) expression of katG::lacZ in strain BW25113 (wt); (c) expression of katG::lacZ in mutants for thiol redox systems (*R < 0.01 between the values of parameters in growing and starving cultures for each strain); (d) expression of katE::lacZ in strain BW25113 (wt); (e) expression of iucC::lacZ in strains BW25113 (wt) and JW2663 (gshA); (f) expression of sulA::lacZ in the studied mutants during phosphate starvation.
The decreased extracellular H2O2 levels observed during phosphate starvation may be the result of both a decrease in peroxide production and an increase in the activity of hydrogen peroxide degrading enzymes such as catalases G and E. The gene expression of katG, which encodes catalase G, is regulated by the transcription factor OxyR, which is activated by H2O2, and the regulator of the general stress response RpoS during the transition to the stationary phase [30]. katE, which encodes catalase E, is expressed under the control of RpoS, whose level of is inversely proportional to the specific growth rate [31].
When growing on phosphate, the expression of katG::lacZ was maintained at a constant level, but rapidly increased with the onset of starvation (Fig. 3b). The addition of phosphate reduced expression of katG to the level observed in growing culture. In a growing culture, expression of katG::lacZ in single mutants was higher than in the parental strain by an average of 25%, and in double mutants gshA trxA and gor trxB by 5.6 and 2.7 times, respectively (Fig. 3c). Fasting for 1 hour led to an increase expression of katG::lacZ in all strains, including the parent strain by 76%, and in single mutants by an average of 47%. Double mutants retained a high level of expression of katG::lacZ. It is noteworthy that in double mutants grown with phosphate and starved for 1 h, low levels of H2O2 corresponded to the highest expression of katG::lacZ. Although 24 h after the onset of fasting, the expression level of katG::lacZ in all strains slightly decreased compared to the values observed after 1 h of starvation, it still remained higher than in bacteria growing on a medium with phosphate (Fig. 3c). A fasting-induced increase in the expression of katE::lacZ (Fig. 3d) occurred. In a phosphate-containing culture of the parental E. coli strain the expression of this fusion increased by 17% in 2 h (from 8852 ± 851 to 10 377 ± 72 Miller units), while in the starving culture it increased by 86% (up to 16486 ± 220).
The major cause of DNA damage and death E. coli under peroxide stress was the formation of highly toxic hydroxyl radicals in the Fenton reaction [32]:
The free iron pool in E. coli was maintained at a low level and was strictly controlled by the Fur transcriptional regulator [33]. One of the members of the Fur regulon is iucC, which encodes a protein involved in the synthesis of siderophore aerobactin. A decrease in the level of intracellular free iron stimulates the expression of iucC. Using iucC::lacZ [22] makes it possible to trace the degree of induction of the Fur regulon and obtain an indirect estimate of the state of the free iron pool. As can be seen from Fig. 3e, in the parent E. coli strain and the gshA mutant, after 2 hours of fasting, the expression of iucC::lacZ rose by 21% compared to cells growing on phosphate (p < 0.05). This may indicate a slight decrease in the content of free iron in the cytoplasm.
In response to DNA damage E. coli induce the SOS regulon that controls the genes involved in DNA repair [34]. To study the expression of SOS genes, we used a transcriptional fusion of sulA, a mediator of filamentation in the SOS response, with lacZ [21]. In cultures of E. coli growing on a medium with phosphate, the highest level of expression of sulA::lacZ occurred in the gshA and gshA trxA strains (215 ± 4 Miller units), which is 32% higher than in the parental strain (163 ± 4) (Fig. 3f). The lowest expression was in the gor strain (130 ± 1). All strains (including the parent) showed a statistically significant decrease in sulA::lacZ expression during 24 h of phosphate starvation, by an average of 26%, which may indicate a decrease in DNA damage. After 48 h of fasting, in all the studied strains, the expression level of sulA::lacZ slightly increased compared to the 24-h point (by a maximum of 25% in the mutant gshA), but did not reach the values typical for a growing culture.
The Effect of Phosphate Starvation on the Level and Redox Status of Glutathione in E. coli Cells
We have previously shown that the levels of intracellular (GSHin) and extracellular (GSHout) of glutathione, as well as the redox status of glutathione, change significantly under various stresses [3, 5, 14, 35, 36]. It was of interest to test the effect of phosphate starvation on these parameters.
In cells growing on phosphate E. coli had the highest concentration of GSHin found in the gor mutant (11.1 ± 0.07 µM/OD600), the smallest occurred in the trxA mutant (3.3 ± 0.6). The gor trxB, trxB mutants and the parent strain contained 4.7 ± 0.4, 7.2 ± 0.2, and 7.7 ± 0.3 μM/OD600, respectively. After 1 h of cultivation in a medium without phosphate, all strains showed a statistically significant (p < 0.05) increase in GSHin. The smallest increase (by 1.6 times) occurred in the parent strain, the largest occurred in gor trxB (4.4 times) (Fig. 4a). Elevated GSHin persisted in all strains after 24 h of starvation. By 48 h of starvation, all strains showed a tendency to decrease in intracellular GSH, although to a different extent. In the parent strain, as well as in trxA and trxB mutants the GSHin level still remained higher than in cultures growing on phosphate, and in gor and gor trxB it approached this value (Fig. 4a).
It is known that E. coli accumulate glutathione in micromolar concentrations in the medium [37]. The level of extracellular glutathione depends on the cultivation conditions and can change significantly in stressful situations [3]. We have shown that during growth on a medium with phosphate, the level of GSHout was maintained in the range from 1.2 ± 0.15 (trxA) to 3.0 ± 0.03 (gor) µM/OD600 and correlated with the values of GSHin (r = 0.95). Phosphate starvation in all strains was accompanied by a decrease in GSHout levels, which was faster during the first hour (Fig. 4d). By this time and further, until the end of cultivation, the differences in GSHout between strains sharply decreased. After 48 h of fasting, the GSHout approached trace amounts (Fig. 4d). As follows from the comparison of the data presented in Figs. 4a and 4d, a significant contribution to the increase in the GSHin pool in the process of starvation can come from the medium.
The level of intracellular oxidized glutathione (GSSGin) in the medium with phosphate in the parental strain was 0.16 ± 0.02 µM/OD600 (Fig. 4b). GSSGin concentrations close to this value were found in gor, trxA, and trxB strains: 0.20 ± 0.06, 0.19 ± 0.04, and 0.19 ± 0.01, respectively. Mutants lacking both gor trxB reductases were markedly different from other strains, with a level of GSSGin that was twice as high as that of the parent strain (p < 0.05). There was no statistically significant change in the concentration of GSSGin during starvation in all strains except gor trxB. In this strain, 1 hour after the onset of starvation, the level of GSSGin increased by 2.3 times (p < 0.05), and then, during further cultivation, it decreased to a level close to the initial value (Fig. 4b).
In strains growing in the presence of phosphate, the concentration of oxidized extracellular glutathione (GSSGout) was on average 3 times lower than GSHout (Fig. 4c). In the parent strain and trxA and trxB mutants a low level of GSSGout was also preserved during starvation. The exception was the gor trxB mutant, whose concentration of GSSGout in cells growing on phosphate was only 1.5 times lower than GSHout, while during starvation it increased by 3.6 times, reaching the level of GSHin, which was observed in the growing culture and after 48 hours of starvation. For the gor mutant the fasting level of GSSGout almost doubled (Fig. 4e).
The redox status of glutathione reflects the GSH/GSSG ratio. Due to the fact that the initial levels of GSSGin all strains changed in a narrow range, differences in GSHin/GSSGin correlated with GSHin, which varied over a wider range (Fig. 4c). In our experiments in a medium with phosphate, the GSHin/GSSGin for the parent strain was 48.6 ± 3.9. The absence of both reductases in the gor trxB strain sharply reduced this value by more than three times. During the phosphate starvation, there was a significant increase in GSHin/GSSGin towards reductive values that reached a maximum for gor after 1 hour (87 ± 6), and for trxA, trxB, and the parent strain (77 ± 13, 114 ± 6 and 110 ± 19, respectively) at 24 h after the start of cultivation (Fig. 4c). The smallest increase was observed in gor trxB (32 ± 10), which is associated with the lowest content of GSHin among other strains and the largest in GSSGin. In all strains growing on phosphate, GSHout/GSSGout changed from 1.4 ± 0.16 for gor trxB to 4.6 ± 0.3 in the parent strain, averaging about 3.3, which is 10 times less than GSHin/GSSGin. In contrast to GSHin/GSSGin, GSHout/GSSGout in all strains significantly decreased towards oxidative values during starvation (Fig. 4f).
It should be noted that the GSHin/GSSGin values obtained in this work were significantly lower than those observed earlier when growing E. coli BW25113 on M9 medium, where this ratio varied from 330 to 500 under normal growth conditions [14]. In our work, in the same bacteria growing on the MOPS medium, the ratio of GSHin/GSSGin was about 5 times lower, which is associated with an increase in the level of GSSGin. This increase in GSSGin may indicate a higher intensity of oxidative processes in the MOPS medium, where the concentration of Fe2+ ions was 5 times more than in M9.
DISCUSSION
An increase in ROS production, activation of components of antioxidant systems, and oxidative damage to biomolecules, together with a decrease in survival or growth rate, are usually considered as indicators of oxidative stress in the response of bacteria to stresses that are not directly related to the action of ROS. As a result of studying the dynamics of changes in the oxygen content in the medium, we showed that after the depletion of phosphate in the presence of glucose, the bacteria retained their respiratory activity, although at a lower level than those growing with phosphate.
Under aerobic conditions, E. coli produce superoxide and H2O2 when molecular oxygen randomly receives electrons from reduced flavoprotein cofactors. Other, including extracellular, sources of ROS are also possible [38]. Under the conditions we chose, the transition to phosphate starvation was accompanied by a decrease in the accumulation of H2O2 in the medium of all studied strains. This could be a consequence of a decrease in the intensity of respiration, as evidenced by the increase in oxygen content. However, an increase in the rate of superoxide production and gene expression of katG and katE during the transition to starvation indicates an increase in ROS production. Expression of both genes is controlled by RpoS, which responds to a decrease in growth rate and transition to the stationary phase [30]. Expression of katG may also be controlled by the transcriptional regulator OxyR, which responds to an increase in intracellular H2O2 and apart from katG activates transcription of other genes (ahpFC, gorA, etc.) involved in the protection of E. coli from peroxide stress [39]. Thus, the decrease in the accumulation of H2O2 in the medium observed during phosphate starvation may be due to the induction of antioxidant enzymes.
The significant changes in the redox status of glutathione discovered during phosphate starvation are of particular interest. The most important ones are associated with a decrease in the level of GSH in the medium and with an increase in its content in the cytoplasm, as well as with a shift in GSHin/GSSGin towards reductive values, and GSHout/GSSGout towards oxidative values.
Earlier, an increase in GSHin was found in E. coli growing under aerobic conditions on M9 medium, under the action of ciprofloxacin [40, 41] and chloramphenicol [14], in amino-acid starvation [14], and in glucose depletion [35]. In addition, under the action of ciprofloxacin and chloramphenicol, an increase in GSHout was simultaneously observed, whereas during amino acid starvation, changes in GSHout were absent. The changes in the status of glutathione during phosphate starvation we revealed are similar to those that occurred during growth arrest of E. coli due to glucose depletion when there was an increase in GSHin at the same time and a decrease in GSHout [35]. Previously, it was shown that factors that reduce the energy status of cells inhibit the GSH transmembrane cycle and stimulate GSH are imported from the medium [5]. This may explain the similar changes in glutathione status inside and outside the cell when glucose and phosphate are depleted. In both cases, fasting was accompanied by a significant decrease in the level of ATP [35, and this work]. An increase in the ATP level was noted under the action of ciprofloxacin [41] and chloramphenicol [42].
In the works cited above, it was established that stress-induced changes in the status of glutathione are associated with its participation in cysteine homeostasis. Bacteria maintain a low level of intracellular free cysteine due to its high redox activity and ability to reduce intracellular iron, thereby supplying a substrate for the Fenton reaction [15, 16]. GSH is a weak iron reducer [15], thus the incorporation of cysteine into the glutathione molecule serves as one of the effective mechanisms for maintaining cysteine homeostasis with an increase in its intracellular concentration, which is observed when protein synthesis is stopped [14]. Reducing the excess of cysteine in cells is also achieved by its export to the medium and desulfurization with the formation of H2S [14, 16, 17]. The increase in GSHin levels we observed during phosphate starvation; it can also be a consequence of the intensification of glutathione synthesis when an excess of cysteine occurs due to inhibition of protein synthesis and other metabolic processes that consume cysteine.
It is known that redox systems of glutathione and thioredoxin largely functionally overlap and duplicate each other. As a result, the absence of components of one of the systems often does not manifest itself phenotypically, and only multiple mutations affecting both systems lead to significant metabolic disorders. In addition, the redox systems of glutathione and thioredoxin closely interact with the OxyR regulon, which is activated by H2O2. Thus, under peroxide stress, the loss of glutathione reductase or thioredoxin reductase can be compensated by an increase in the expression of katG (and GSH synthesis in the first case) [6].
The behavior of single mutants under phosphate starvation generally corresponds to what was observed earlier under peroxide stress. According to such an integral indicator as the growth rate, mutants (with the exception of gshA) differed insignificantly from the parental strain and from each other. The data on the gor trxB double mutant are of particular interest. By comparison with the parental and single mutants, this strain was characterized by many times higher expression of katG::lacZ, the highest levels of oxidized intra- and extracellular glutathione and, accordingly, the lowest GSH/GSSG ratio in both compartments. After 48 h of phosphate starvation, this strain (as well as at gshA trxA) had the lowest CFU. The double mutant gshA trxA had similar properties (excluding indicators related to glutathione status). The data on the behavior of the gor trxB mutant provide additional evidence for the presence of oxidative stress in phosphate starvation. Considering all the listed properties, the gor trxB double mutant can be considered as a convenient model for studying the response of bacteria to stresses not associated with the direct action of ROS.
The results we obtained are summarized in Fig. 5.
Based on the results we obtained, it can be said that during phosphate starvation in E. coli the rate of ROS production increases, but the induction of catalase prevents the accumulation of H2O2 above the toxic level. Combined with low levels of free iron, this allows bacteria to maintain conditions that reduce the risk of DNA damage, as evidenced by the lack of upregulation of the sulA gene in starved cells, included in the SOS regulon. It is also noteworthy that a significant decrease in the ATP pool, which is observed as the cell growth rate decreases, is not accompanied by a large drop in the membrane potential and complete outflow of potassium, which may indicate the preservation of normal membrane activity in starving cells. Phosphate starvation causes a significant shift in the status of glutathione inside and outside cells, which, apparently, reflects the work of the mechanisms of intracellular cysteine homeostasis. The totality of the ongoing changes leads to the fact that under the described conditions, E. coli can maintain high viability for a long time, allowing it to quickly resume growth after the introduction of phosphate into the medium. Mutations in individual components of thiol redox systems do not lead to critical disorders causing cell death due to coordinated changes in the activities of other antioxidant systems.
REFERENCES
Sevilla E., Bes M.T., Gonzalez A., Peleato M.L., Fillat M.F. 2019. Redox-based transcriptional regulation in prokaryotes: Revisiting model mechanisms. Antioxid. Redox Signal. 30, 1651–1696. https://doi.org/10.1089/ars.2017.7442
Imlay J.A. 2008. Cellular defenses against superoxide and hydrogen peroxide. Ann. Rev. Biochem. 77, 755–776. https://doi.org/10.1146/annurev.biochem.77.061606.161055
Smirnova G.V., Oktyabrsky O.N. 2005. Glutathione in bacteria. Biochemistry (Moscow). 70, 1199–1211.
Vlamis-Gardikas A. 2008. The multiple functions of the thiol-based electron flow pathways of Escherichia coli: Eternal concepts revised. Biochim. Biophys. Acta. 1780, 1170–1200. https://doi.org/10.1016/j.bbagen.2008.03.013
Smirnova G., Muzyka N., Oktyabrsky O. 2012. Transmembrane glutathione cycling in growing Escherichia coli cells. Microbiol. Res. 167, 166–172. https://doi.org/10.1016/j.micres.2011.05.005
Carmel-Harel O., Storz G. 2000. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 54, 439–461. https://doi.org/10.1146/annurev.micro.54.1.439
Wanner B.L. 1996. Phosphorus assimilation and control of the phosphate regulon. In Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt F.C., Curtiss III R., Ingraham J.L., Lin E.C.C., Low K.B., Magasanik B., Reznikoff W.S., Riley M., Schaechter M., Umbrager H.E., Eds. Washington DC: Am. Soc. Microbiol., 1357–1381.
Lamarche M.G., Wanner B.L., Crepin S., Harel J. 2008. The phosphate regulon and bacterial virulence: A regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 32 (3), 461–473. https://doi.org/10.1111/j.1574-6976.2008.00101.x
VanBogelen R.A., Olson E.R., Wanner B.L., Neidhardt F.C. 1996. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J. Bacteriol. 178 (15), 4344–4366. https://doi.org/10.1128/jb.178.15.4344-4366.1996
Gerard F., Dri A.M., Moreau P.L. 1999. Role of Escherichia coli RpoS, LexA and H-NS global regulators in metabolism and survival under aerobic, phosphate-starvation conditions. Microbiology. 145, 1547–1562. https://doi.org/10.1099/13500872-145-7-1547
Moreau P.L., Gerard F., Lutz N.W., Cozzone P. 2001. Non-growing Escherichia coli cells starved for glucose or phosphate use different mechanisms to survive oxidative stress. Mol. Microbiol. 39, 1048–1060. https://doi.org/10.1046/j.1365-2958.2001.02303.x
Moreau P.L. 2004. Diversion of the metabolic flux from pyruvate dehydrogenase to pyruvate oxidase decreases oxidative stress during glucose metabolism in nongrowing Escherichia coli cells incubated under aerobic, phosphate starvation conditions. J. Bacteriol. 186, 7364–7368. https://doi.org/10.1128/JB.186.21.7364-7368.2004
Yuan Z.C., Zaheer R., Finan T.M. 2005. Phosphate limitation induces catalase expression in Sinorhizobium meliloti, Pseudomonas aeruginosa and Agrobacterium tumefaciens. Mol. Microbiol. 58 (3), 877–894. https://doi.org/10.1111/j.1365-2958.2005.04874.x
Smirnova G.V., Tyulenev A.V., Bezmaternykh K.V., Muzyka N.G., Ushakov V.Y., Oktyabrsky O.N. 2019. Cysteine homeostasis under inhibition of protein synthesis in Escherichia coli cells. Amino Acids. 51, 1577–1592. https://doi.org/10.1007/s00726-019-02795-2
Park S., Imlay, J.A. 2003. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 185, 1942–1950. https://doi.org/10.1128/JB.185.6.1942-1950.2003
Imlay K.R.C., Korshunov S., Imlay J.A. 2015. The physiological roles and adverse effects of the two cystine importers of Escherichia coli. J. Bacteriol. 197, 3629–3644. https://doi.org/10.1128/JB.00277-15
Korshunov S., Imlay K.R.C., Imlay J.A. 2020. Cystine import is a valuable but risky process whose hazards Escherichia coli minimizes by inducing a cysteine exporter. Mol. Microbiol. 113, 22–39. https://doi.org/10.1111/mmi.14403
Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K.A., Tomita M., Wanner B.L., Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2, 2006.0008. https://doi.org/10.1038/msb4100050
Tao K., Makino K., Yonei S., Nacata A., Shinagawa H. 1989. Molecular cloning and nucleotide sequencing of oxyR, the positive regulatory gene of a regulon for an adaptive response to oxidative stress in Escherichia coli: Homologies between OxyR protein and a family of bacterial activator proteins. Mol. Gen. Genet. 218, 371–376. https://doi.org/10.1007/bf00332397
Mulvey M.R., Switala J., Borys A., Loewen P.C. 1990. Regulation of transcription of katE and katF in Escherichia coli. J. Bacteriol. 172, 6713–6720. https://doi.org/10.1128/jb.172.12.6713-6720.1990
Volkert M.R., Gately F.H., Hajec L.I. 1989. Expression of DNA damage-inducible genes of Escherichia coli upon treatment with methylating, ethylating and propylating agents. Mutation. Res. 217, 109–115. https://doi.org/10.1016/0921-8777(89)90062-1
Maringanti S., Imlay J.A. 1999. An intracellular iron chelator pleiotropically suppresses enzymatic and growth defects of superoxide dismutase-deficient Escherichia coli. J. Bacteriol. 181, 3792–3802. https://doi.org/10.1128/JB.181.12.3792-3802.1999
Neidhardt F.C., Bloch P.L., Smith D.F. 1974. Culture medium for enterobacteria. J. Bacteriol. 119, 736–747. https://doi.org/10.1128/jb.119.3.736-747.1974
Wickens H.J., Pinney R.J., Mason D.J., Gant V.A. 2000. Flow cytometric investigation of filamentation, membrane patency and membrane potential in Escherichia coli following ciprofloxacin exposure. Antimicrob. Agents Chemother. 44, 682–687. https://doi.org/10.1128/AAC.44.3.676-681.2000
Smirnova G.V., Muzyka N.G., Ushakov V.Y., Tyulenev A.V., Oktyabrsky O.N. 2015. Extracellular superoxide provokes glutathione efflux from Escherichia coli cells. Res. Microbiol. 166, 609–617. https://doi.org/10.1016/j.resmic.2015.07.007
Korshunov S., Imlay J.A. 2006. Detection and quantification of superoxide formed within the periplasm of Escherichia coli. J. Bacteriol. 188, 6326–6334. https://doi.org/10.1128/JB.00554-06
Seaver L.C., Imlay J.A. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183, 7173–7181. https://doi.org/10.1128/JB.183.24.7173-7181.2001
Tietze F. 1969. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 27, 502–522. https://doi.org/10.1016/0003-2697(69)90064-5
Miller J.H. 1972. Experiments in Molecular Genetics. Cold Spring Harbor, New York: Cold Spring Harbor Lab. Press.
Ivanova A., Miller C., Glinsky G., Eisenstark A. 1994. Role of the rpoS(katF) in oxyR independent regulation of hydroperoxidase I in Escherichia coli. Mol. Microbiol. 12, 571–578. https://doi.org/10.1111/j.1365-2958.1994.tb01043.x
Ihssen J., Egli T. 2004. Specific growth rate and not cell density controls the general stress response in Escherichia coli. Microbiology. 150, 1637–1648. https://doi.org/10.1099/mic.0.26849-0
Imlay J.A., Linn S. 1988. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 240, 640–642. https://doi.org/10.1126/science.2834821
Hantke K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4, 172–177. https://doi.org/10.1016/s1369-5274(00)00184-3
Maslowska K.H., Makiela-Dzbenska K., Fijalkowska I.J. 2019. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 60, 368–384. https://doi.org/10.1002/em.22267
Tyulenev A.V., Smirnova G.V., Muzyka N.G., Ushakov V.Y., Oktyabrsky O.N. 2018. The role of sulfides in stress-induced changes of Eh in Escherichia coli cultures. Bioelectrochemistry. 121, 11–17. https://doi.org/10.1016/j.bioelechem.2017.12.012
Smirnova G.V., Tyulenev A.V., Muzyka N.G., Oktyabrsky O.N. 2018. The sharp phase of respiratory inhibition during amino acid starvation in Escherichia coli is RelA-dependent and associated with regulation of ATP synthase activity. Res. Microbiol. 169, 157–165. https://doi.org/10.1016/j.resmic.2018.02.003
Owens R.A., Hartman P.E. 1986. Export of glutathione by some widely used Salmonella typhimurium and Escherichia coli strains. J. Bacteriol. 168, 109–114. https://doi.org/10.1128/jb.168.1.109-114.1986
Imlay J.A. 2013. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 11, 443–454. https://doi.org/10.1038/nrmicro3032
Aslund F., Zheng M., Beckwith J., Storz G. 1999. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc. Natl. Acad. Sci. U. S. A. 96, 6161–6165. https://doi.org/10.1073/pnas.96.11.6161
Smirnova G.V., Tyulenev A.V., Muzyka N.G., Peters M.A., Oktyabrsky O.N. 2017. Ciprofloxacin provokes SOS-dependent changes in respiration and membrane potential and causes alterations in the redox status of Escherichia coli. Res. Microbiol. 168, 64–73. https://doi.org/10.1016/j.resmic.2016.07.008
Smirnova G.V., Tyulenev A.V., Muzyka N.G., Oktyabrsky O.N. 2022. Study of the contribution of active defense mechanisms to ciprofloxacin tolerance in Escherichia coli growing at different rates. Antonie Van Leeuwenhoek. 115, 233–251. https://doi.org/10.1007/s10482-021-01693-6
Smirnova G., Tyulenev A., Muzyka N., Ushakov V., Samoilova Z., Oktyabrsky O. 2023. Influence of growth medium composition on physiological responses of Escherichia coli to the action of chloramphenicol and ciprofloxacin. BioTech. 12, 43. https://doi.org/10.3390/biotech12020043
Funding
The study was carried out with the financial support of the Russian Science Foundation (grant no. 22-14-00093).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving human participants or animals as research subjects.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations. ROS, reactive oxygen species; GSH (L-γ-glutamyl-L-cysteinyl-glycine), reduced form of glutathione; GSSG, oxidized form of glutathione; in/out, in the cytoplasm/in the culture medium; SOD, superoxide dismutase.
Rights and permissions
About this article
Cite this article
Smirnova, G.V., Tyulenev, A.V., Muzyka, N.G. et al. Changes in the Activity of Antioxidant Systems of Escherichia coli under Phosphate Starvation. Mol Biol 57, 965–977 (2023). https://doi.org/10.1134/S0026893323060171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893323060171