Abstract
Cephalotaxus mannii Hook f. is a rare medicinal plant used for leukemia treatment, however its fungal endophytes as a promising resource of pharmaceutical compounds remain poorly characterized. The present study is the first research on endophytic fungi associated with C. mannii collected in Vietnam and their potent biological activities. A total of 18 endophytic fungi were isolated; according to the results of morphological and internal transcribed spacer (ITS) sequence analyses, they belonged to six genera: Penicillium, Fusarium, Aspergillus, Diaporthe, Megasporoporia, and Trichoderma. Among these strains, the ethyl acetate extracts of Penicillium citrinum WDF8 and Fusarium perseae WDF12 showed remarkable antibacterial activity against at least 5 tested bacteria. Of note, only the WDF12 extract displayed potential cytotoxicity against A549 and MCF7 cancer cell lines with IC50 values at 13.4 ± 0.9 and 17.8 ± 2 µg/mL, respectively. Further cytotoxicity evaluation led to the identification of 10-deacetylbaccatin III-10-O-acetyl transferase (dbat) gene essential for the biosynthesis of the anti-cancer drug paclitaxel in the fungal strain WDF8. Both extracts also showed strong antioxidant activity against DPPH, hydroxyl, and superoxide anion radicals, attributed to a high level of polyphenols and flavonoids present. This study proved that endophytic fungi from C. mannii exhibit excellent antibacterial, anticancer, and antioxidant activities and may be promising candidates for the production of paclitaxel and new compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Fungal endophytes are known act as inter- and intracellular colonizers of plant tissues for all or part of their life cycle without signs of disease or morphological changes to the plant life cycle. During symbiosis, fungal endophytes help the host resist pathogens as well as external biotic and abiotic stresses, while the plant supports the endophytes by providing nutrients and shelter (Toghueo, 2020). Notably, these endophytes are capable of producing secondary metabolites similar to the host plants. For example, paclitaxel obtained from the bark of Taxus brevifolia was also produced by endophytic fungus Taxomyces andreanae (Stierle et al., 1993). This was attributed to horizontal gene transfer. Intense attempts to investigate paclitaxel-producing fungi have led to an interesting finding that paclitaxel is not only secreted by fungi derived from the Taxus species, but also from other medicinal plants, such as Ginko biloba and Terminalia arjuna (Abdel-Fatah et al., 2021). Given that baccatin III known as a precursor of paclitaxel is synthesized by 10‑deacetylbaccatin III-10-O-acetyl transferase (DBAT), the dbat gene is used as molecular markers to screen for paclitaxel-producing fungi.
Apart from plant-derived compounds, fungal endophytes are also a rich source of secondary metabolites with medicinal and pharmaceutical applications. Frequently found in medicinal plants, Colletotrichum, Fusarium, Alternaria, Penicillium, and Aspergillus exhibit significant activity against microbial pathogens, oxidative stress, and cancer (da Silva et al., 2020; Toghueo, 2020). Fusarium proliferatum isolated from Dysoxylum binectariferum Hook.f (Meliaceae) could produce rohitukine, a chromane alkaloid exhibiting cytotoxicity toward HCT-116 and MCF-7 human cancer cell lines (Mohana et al., 2012). The endophytic fungus Penicillium citrinum BCC71086 isolated from Tamarindus indica was found to secrete 22 antitubercular metabolites, including 7 novel and 15 known compounds (Dramae et al., 2022). Reactive oxygen species and other free radicals are related to the emergence of cancer rising the need for natural antioxidants (Carocho and Ferreira, 2013). Cajaninstilbene acid extracted from endophytic Fusarium solani ERP-07, Fusarium oxysporum ERP-10, and F. proliferatum, was reported as an antioxidant (Toghueo, 2020).
Cephalotaxus mannii Hook f. is a rare medicinal plant occurring in China, India, and southeastern Asia, including Vietnam. Being listed as vulnerable in The International Union for Conservation of Nature (IUCN) Red List, C. mannii harbors various bioactive compounds, including cephalotaxine, isoharringtonine, norisoharringtonine, desoxyharringtonine, nordesoxyharringtonine, 3-epi-schellhammericine, homoharringtonine, and isoharringtonine, among which homoharringtonine and isoharringtonine are of importance for the leukemia treatment. Earlier work reported only the isolation and identification of endophytic fungi from C. mannii collected in Thailand and China (Saithong et al., 2010). Another study revealed the antimicrobial activity of fungal endophytes isolated from Cephalotaxus hainanensis Li (Yang et al., 2015). The present study focused for the first time on the isolation and evaluation of the medicinal potential of fungal endophytes recovered from C. mannii collected in northern Vietnam. The findings underline the importance of fungal endophytes and provide potential fungal candidates to characterize the promising bioactive compounds at molecular and mechanistic levels in near future.
MATERIALS AND METHODS
Sample collection and isolation of endophytic fungi. The leaf, stem, and root samples of Cephalotaxus mannii Hook f. were collected in Dong Van (23°15′30′′ N 105°17′24′′ E), Ha Giang Province, northern Vietnam in March 2020; no specific permission was required for the location. With the help of expert plant gatherers and local ethnic minority peoples, two healthy plants were collected in the same vicinity. Each sample was separately kept in a sterile polymer bag and delivered to the laboratory of the Institute of Biotechnology, Vietnam Academy of Science and Technology. The plant samples were identified and preserved by the Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology. The collected plant specimen surface was sterilized following the procedure previously described (Vu et al., 2022). Briefly, after washing in running tap water to remove debris, the plant segments were sequentially immersed in 70% ethanol for 30 s, 3.5% sodium hypochlorite solution for 2 min, 70% ethanol for 2–5 s, and rinsed with sterile distilled water. Then, the samples were cut into small pieces (~1.0 × 0.5 cm) and placed in 9-cm diameter petri dishes (6 pieces/plate) containing potato dextrose agar (PDA) supplemented with 100 mg/L streptomycin to suppress bacterial contamination. Petri dishes were incubated at 28°C for 7−10 days, and the samples were frequently checked for visual growth of fungi. The hyphal type of colonies was inoculated onto fresh PDA plates for purification. All fungal strains were stored in 15% glycerol (vol/vol) at ‒80°C for long-term preservation.
Fungal identification. The fungal isolates were individually cultured in PDA plates for observation of hyphal growth, colony morphology, and pigment production according to a previous report (Ngo et al., 2021). Mycelia, conidiophores, and conidia were examined under a light microscope at 40× objective (Olympus, Japan). Genomic DNA was isolated using a QIAamp DNA mini kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. The primers ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') and PCR amplification conditions were employed to amplify the Internal Transcribed Spacer (ITS) gene (Ngo et al., 2021). The ITS sequences were compared with existing sequences in the GenBank database (NCBI) using BLASTn search and then deposited in GenBank. The phylogenetic analysis was performed using MEGA v11.0 by the maximum likelihood Bootstrap method (Kumar et al., 2016). The reliability of the phylogenetic tree was tested by bootstrap analysis using 1000 replicates and Cunninghamella elegans CBS 160.28T (NR_154747) was used as an out-group.
Obtaining the crude fungal extract. Fungal isolates were cultivated in Erlenmeyer flasks containing Potato Dextrose Broth (PDB) and incubated in the dark at 28°C under orbital agitation of 150 rpm. After 14 days of incubation, fungal biomass was removed by vacuum filtration and the culture filtrates were extracted with a double volume of ethyl acetate in a separatory funnel (Vu et al., 2022). The solvent phase was collected and evaporated at 45°C to obtain the crude extract. The crude extract was dissolved in 1% (vol/vol) dimethyl sulfoxide (DMSO) for antimicrobial and cytotoxic experiments, while 70% ethanol was used for evaluating the antioxidant activities.
Antimicrobial screening. The antimicrobial activity of crude extracts from endophytic fungi was examined against two gram-negative bacteria (Escherichia coli ATCC 11105 and Pseudomonas auroginosa ATCC 9027), four gram-positive bacteria (Bacillus cereus ATCC 11778, methicillin-resistant Staphylococcus epidermidis (MRSE) ATCC 35984, methicillin-resistant Staphylococcus aureus (MRSA) ATCC 33591, and Enterococcus faecalis ATCC 29212), and one yeast Candida albicans ATCC 10231 using the agar well diffusion method (Gonelimali et al., 2018). The test microorganisms were spread on the nutrient agar medium in 9-cm diameter petri dishes and 100 μL of each extract (100 μg/mL) was added to every well. Antimicrobial activity was expressed as the zone of inhibition (mm).
Screening of the anticancer activity. The cytotoxic potential of crude extracts was evaluated by using the sulforhodamine B (SRB) test with the human lung cancer A549 and human breast adenocarcinoma MCF7 cell lines (Skehan et al., 1990). The human lung cancer A549 and human breast adenocarcinoma MCF7 cell lines were grown on 96-well plates with starting density of around 104 cells and incubated at 37°C, 5% CO2, and 95% humidity for 24 h. Fungal extracts were added at different concentrations and left for 24 h before the cells were fixed with cold 10% (wt/vol) trichloroacetic acid at 4°C for 1 h. The plates were examined (OD540) in a BioTek EXL800 microplate reader. The positive and negative controls were ellipticine and 0.1% DMSO (vol/vol), respectively. The IC50 was defined as the sample concentration that resulted in 50% of cancer cell survival by comparison with a control conducted in identical conditions.
Evaluation of the antioxidant activity. The 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity was evaluated according to a previous protocol (Kadaikunnan et al., 2015). About 100 µL of 70% ethanol extract prepared in different concentrations (0.1, 0.2, 0.4, and 0.6 mg/mL) was mixed with 100 µL of 0.1 mM DPPH and left for 30 min in the dark at room temperature. The absorbance was measured at 517 nm and deionized water was used as a blank. The ability to scavenge free hydroxyl radical was assessed at an optical density of 624 nm as described previously (Kadaikunnan et al., 2015). Briefly, the reaction mixture contained 1.0 mL of 0.5 mM FeSO4, 0.5 mL of 0.435 mM brilliant green, and 0.75 mL of 3% H2O2. The reaction was started by addition of 70% ethanol extract with concentrations ranging from 0.1 to 0.6 mg/mL, followed by incubation at 37°C for 30 min. The superoxide scavenging activity was assessed following the procedure described previously (Vu et al., 2022). About 900 µL of 0.05 M Tris–HCl (pH 8.2) was added to 80 µL of 2.5 mM pyrogallol, which was subsequently mixed with 200 µL of crude extract (0.1; 0.2; 0.4; 0.6 mg/mL). The reaction was carried out at room temperature for 5 min and then measured at 299 nm.
Determination of the total polyphenol and flavonoid content. To detect total polyphenol content in the fungal extracts, the Folin-Ciocalteu colorimetric method with a slight modification was applied (da Silva et al., 2020). The reaction mixture containing 20 µL of 70% ethanol extract and 100 µL Follin-Ciocalteu reagent was incubated for 5 min at room temperature. After that, 80 µL of 4% (wt/vol) sodium carbonate was added to the mixture and absorbance was measured at 765 nm. Results were calculated as µg gallic acid equivalents per g of fungal extract (µg GAE/mg) based on a gallic acid calibration curve. The total flavonoid content was spectrophotometrically evaluated using a colorimetric method (da Silva et al., 2020). Briefly, the reaction mixture comprised 10 µL of 5% (wt/vol) NaNO2, 10 µL of 10% (wt/vol) AlCl3, 60 µL of 1 M NaOH, 120 µL of distilled water, and 30 µL of fungal extract. After incubating at room temperature for 30 min, the absorbance was read at 510 nm. The total flavonoid content was represented as µg quercetin equivalents per g of fungal extract (µg QE/mg).
PCR-based molecular screening for paclitaxel-producing fungi using the dbat. Given that the gene dbat coding for 10-deacetylbaccatin III-10-O-acetyl transferase catalyzes the formation of baccatin III, the immediate diterpenoid precursor of paclitaxel (Zhang et al., 2008; Kumar et al., 2019), dbat was employed as a molecular marker to screen paclitaxel-producing fungi. The specific primers dbat-F (5'-ATGGCTGACACTGACCTCTCAGT-3'), dbat-R (5'-GGCCTGCTCCTAGTCCATCACAT-3') were used to amplify dbat according to a previous publication (Das et al., 2017). PCR products were analyzed on 2% (wt/vol) agarose gel, purified by a DNA gel extraction kit (Axygen), and sequenced by First BASE Laboratories Sdn. Bhd. (Malaysia). The resulting sequence was compared and aligned with truncated sequences available in the GenBank database, including those of Cladosporium cladosporioides MD-2 (EU375527.1), Aspergillus candidus MD3 (EU883596.3), Lasiodiplodia theobromae SKJM 1101 (KP136287.1) using Jalview v2.11.1.7. To support PCR-based molecular screening results, the fungal strains potentially capable of producing paclitaxel were stained for 1 h with Sudan IV followed by the observation using light microscopy (Soliman and Raizada, 2018).
RESULTS
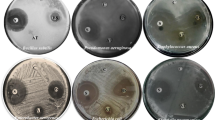
Isolation and identification of endophytic fungi from C. mannii. In total, 18 fungal endophytes with distinct morphology were isolated from C. mannii. Seven fungal isolates (38.9%) were recovered from stems, six (33.3%) were obtained from leaves, and five (27.8%) were derived from roots (Table S1). Based on morphology, microscopic identification, and ITS gene sequence analysis, they were identified as members of 6 genera: Penicillium, Fusarium, Aspergillus, Diaporthe, Megasporoporia, and Trichoderma (Fig. 1, Table S1). Due to support rating of 42%, strains WDF13, WDF15, and WDF20 were identified as members of the genus Diaporthe. Similarly, strain WDF22 was classified as Trichoderma sp. WDF22.
Screening of fungal extracts for antimicrobial activity. Out of 18 endophytic fungi, 7 fungal strains (38.9%), WDF2, WDF4, WDF8, WDF9, WDF11, WDF12, and WDF22, inhibited at least one pathogenic microorganism with inhibition zones ranging from 7.3 ± 0.4 to 26.6 ± 0.4 mm (Table 1). WDF11 extract was active against C. albicans ATCC 10231 only. Among the bioactive extracts, the one from WDF12 showed the best inhibition against P. aeruginosa ATCC 9027 (20.7 ± 0.4 mm), MRSE ATCC 35984 (16.8 ± 0.7 mm), B. cereus ATCC 11778 (14.5 ± 0.4 mm), MRSA ATCC 33591 (26.6 ± 0.4 mm), and E. faecalis ATCC 29212 (26.2 ± 0.4 mm). Despite its lower antimicrobial ability against those pathogens, WDF8 extract had additional activity against E. coli ATCC 11105 with the inhibition zone diameter of 15.2 ± 0.7 mm.
Cytotoxic activity. Only 2 (WDF8 and WDF12) out of 18 extracts showed cytotoxicity against A549 and MCF7 cell lines. The WDF8 extract showed remarkable cytotoxicity against A549 and MCF7 with the IC50 values of 75.7 ± 0.6 and 78.4 ± 0.6 µg/mL, respectively (Table 1). Notably, the cytotoxic activity of the WDF12 extract was around 5 times higher than that of the WDF8 extract.
Further investigation on the cytotoxic property revealed that strain WDF8 had about 500 bp amplified fragment of the dbat gene coding for 10-deacetylbaccatin III-10-O-acetyl transferase. The amplified DNA fragment of dbat was sequenced and analyzed using the NCBI database (Fig. 2a). The dbat sequence of strain WDF8 showed low sequence similarity to those of C. cladosporioides MD-2 (45.1%), A. candidus MD3 (45.9%), and L. theobromae strain SKJM 1101 (45.6%) (Fig. 2b). In addition, paclitaxel production by the fungal strain WDF8 as stained with Sudan IV was observed by light microscopy (Fig. 2c).
Identification of gene dbat of paclitaxel biosynthetic pathway. (a) Amplification of gene dbat, (b) multiple sequence alignment of dbat of paclitaxel-producing fungi aligned with dbat of Penicillium citrinum WDF8, (c) the fungal strain WDF8 stained by Sudan IV to observe resin bodies known as sequestering organelles for fungal paclitaxel.
Antioxidant activity. Since the presence of increasing antioxidant levels has been proved to support cancer prevention (da Silva et al., 2020), the antioxidant properties of potential fungal extracts were evaluated by the reactions of scavenging hydroxyl, DPPH, and superoxide anion radicals. Our results revealed that the WDF8 extract had remarkable hydroxyl radical scavenging activity, which was comparable to that of ascorbic acid at 0.6 mg/mL (Fig. 3a). At lower levels, the WDF12 extract had hydroxyl radical scavenging activity ranging from 15.3 ± 3.5 to 45.2 ± 5.5%. The two mentioned extracts also demonstrated significant antioxidant activity against DPPH: 97.7 ± 3.1 and 95.2 ± 1.3%, respectively, at 0.4 mg/mL (Fig. 4b). The WDF8 extract was found to strongly inhibit superoxide anion radical up to 95.5 ± 2.3% at 0.6 mg/mL, while the WDF12 extract had low superoxide anion scavenging activity at 27.4 ± 4.2% (Fig. 3c).
Antioxidant activity of Penicillium citrinum WDF8 and Fusarium perseae WDF12. Hydroxyl radical (a), DPPH radical (b), superoxide radical (c) scavenging potential of ethyl acetate crude extracts of strains WDF8 and WDF12. Antioxidant activities are represented by triangles along a solid line for the WDF8 extract, black squares along a solid line for the WDF12 extract, and white circles along a solid line for ascorbic acid as positive controls. Phytochemical levels (d) determined in the WDF8 (grey columns) and WDF12 (black columns) extracts.
Total polyphenol and flavonoid contents. High antioxidant activity against free radicals found in WDF8 and WDF12 extracts led to the determination of polyphenol and flavonoid contents. Total polyphenol content of WDF12 was 224.2 ± 4.6 µg GAE/mg extract, which was about 2.1-fold higher than that of WDF8 (Fig. 3d). Along with polyphenols, the total flavonoid content of WDF12 extract was measured as 196.6 ± 3.1 µg GAE/mg extract, while the WDF8 extract contained 75.3 ± 0.6 µg QE/mg extract.
DISCUSSION
Fungal endophytes isolated from medicinal plants are known as potential sources of bioactive compounds that have important applications in biotechnology and medicine. In the present study, 18 fungal endophytes isolated from C. mannii collected in northern Vietnam were identified to belong to 6 genera, among which Penicillium and Fusarium were the most abundant. Penicillium and Fusarium as the most common fungal genera may support C. mannii against insect attack and pathogens, as well as against abiotic and biotic stresses. It was in agreement with previous reports indicating the dominance of Fusarium and Penicillium in plants (Kim et al., 2014; Toghueo, 2020). Different to this result, the most dominant genera found in the bark of C. mannii in northern Thailand and southern China were Geotrichum and Tri-choderma, respectively (Saithong et al., 2010). In the case of C. hainanensis collected in China, the dominant genera were Phomopsis and Colletotrichum (Yang et al., 2015). It seems that fungal species diversity and richness depend on various factors such as sampling locations, geographical coordinates, seasons, ages and types of tissue, and isolation methods.
Among culturable fungi, Megasporoporia minor WDF2 was found for the first time as an endophyte. M. minor is known as a species of crust fungi in the division Basidiomycota described in 2013 (Li and Cui, 2013). The genus Megasporoporia was detected in the fungal community from Paullinia cupana var. sorbilis using the ITS amplicon sequencing but not in the culturable community (Santos et al., 2020). Megasporoporia sp. S47 isolated from a contaminated sediment was reported to efficiently degrade high molecular weight polycyclic aromatic hydrocarbons like benzo(a)pyren (de Lima Souza et al., 2016). Bioactive compounds derived from Megasporoporia spp. have yet not been exploited to date.
C. mannii used for leukemia treatment was found to harbor endophytic fungi with antimicrobial activity. About 38.9% of endophytic fungi from C. mannii showed significant activity against at least one tested pathogen, which was lower than that of C. hainanensis (Yang et al., 2015). Among bioactive strains, Penicillium citrinum WDF8 and Fusarium perseae WDF12 were the most significant fungal sources of antibacterial metabolites, with inhibition zone diameters reaching up to 18.5 ± 0.7 and 26.6 ± 0.4 mm, respectively. The endophytic P. citrinum BCC71086 produced five unknown tanzawaic acid analogs that possessed strong antitubercular activity (Dramae et al., 2022). Scalusamide was reported to exhibit antifungal and antibacterial activities (Tsuda et al., 2005). Since natural selection pressure always interferes with central metabolic pathways and primary metabolite pools of fungi that result in the production of bioactive compounds (Keller, 2019), P. citrinum derived from different ecological niches is still a promising source of new secondary metabolites. In contrast to the overexploitation of P. citrinum, antimicrobial metabolites from F. perseae have not been revealed yet.
Oxidative stress imposed by free radicals or reactive oxygen species contributes to neurodegenerative diseases and cancer (Carocho and Ferreira, 2013). The crude extracts of P. citrinum WDF8 and F. perseae WDF12 also exhibited strong scavenging activity against hydroxyl, superoxide anion, and DPPH radicals at 0.6 mg/mL. Various reports have proved the strong correlation between phytochemicals, including polyphenols and flavonoids, and antioxidant activity (da Silva et al., 2020; Tang et al., 2020). The ethyl acetate crude extract of Nigrospora sphaerica from Euphorbia hirta L. had total phenolic and flavonoid contents of 77.7 ± 0.05 µg GAE/mg and 230.6 ± 2.0 µg RE/mg, respectively, which were responsible for up to 96.8% antioxidant activity (Gautam et al., 2022). The presence of polyphenols and flavonoids in WDF8 extract as the major constituents further supported the assumptions. Despite having around twice higher polyphenol and flavonoid levels, the overall antioxidant activity of F. perseae WDF12 was lower than that of P. citrinum WDF8. It is possible that phytochemicals from the WDF12 extract may exhibit other biological activities rather than antioxidants, which is an interesting subject for future studies.
Endophytic fungi are recognized producers of microbial and plant metabolites that possess cytotoxic activity. In the present study, F. perseae WDF12 extract was highly toxic to both A549 and MCF7 cells with IC50 values of 13.4 ± 0.9 and 17.8 ± 2 µg/mL, respectively. It was in agreement with previous studies proving the potential of Fusarium in finding novel anticancer metabolites such as rohitukine, chloctanspirone, asperphenamate (Mohana et al., 2012; Toghueo, 2020). In the mode of action, 4,6'-anhydrooxysporidinone produced by the endophytic fungus Fusarium lateritium SSF2 inhibited MCF7 cells through the activation of the caspase-9, caspase-7, PARP, and p53 (Lee et al., 2021). In spite of low cytotoxic activity, the presence of the dbat gene was found only in P. citrinum WDF8 that has a potential for paclitaxel production. However, some fungal strains only produce baccatin III instead of paclitaxel, although a positive hit for dbat was detected (Garyali et al., 2013). With more than 354 species in the genus Penicillium, “Penicillium raistrickii, Penicillium chrysogenum, and Penicillium aurantiogriseum” are evidently paclitaxel-producing fungi (Stierle, A.A. and Stierle, D.B., 2000). Thus, P. citrinum WDF8 and F. perseae WDF12 are promising candidates for further chemical profiles exploration in order to uncover novel bioactive compounds.
In summary, this is the first detailed report discovering endophytic fungi from C. mannii collected in Vietnam: P. citrinum WDF8 with a potential of producing paclitaxel; and F. perseae with excellent antibacterial, antioxidant, and cytotoxic activities. This work addresses the missing information of previous research on C. mannii, holds the potential to exploit new bioactive secondary metabolites, and provides leads for the development of the paclitaxel. Further studies are required to characterize pure compounds with antibacterial, antioxidant, and cytotoxic activities at phenotypic and genomic levels.
REFERENCES
Abdel-Fatah, S.S., El-Batal, A.I., El-Sherbiny, G.M., Khalaf, M.A., and El-Sayed, A.S., Production, bioprocess optimization and γ-irradiation of Penicillium polonicum, as a new Taxol producing endophyte from Ginko biloba, Biotechnol. Rep., 2021, vol. 30, p. e00623.
Carocho, M. and Ferreira, I.C., A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives, Food. Chem. Toxicol., 2013, vol. 51, pp. 15−25.
da Silva, M.H.R., Cueva-Yesquén, L.G., Júnior, S.B., Garcia, V.L., Sartoratto, A., de Angelis, D.F., and de Angelis, D.A., Endophytic fungi from Passiflora incarnata: an antioxidant compound source, Arch. Microbiol., 2020, vol. 202, pp. 2779−2789.
Das, A., Rahman, M.I., Ferdous, A.S., Amin, A., Rahman, M.M., Nahar, N., Uddin, M.A., Islam, M.R., and Khan, H., An endophytic basidiomycete, Grammothele lineata, isolated from Corchorus olitorius, produces paclitaxel that shows cytotoxicity, PLoS One, 2017, vol. 12, p. e0178612.
de Lima Souza, H.M., Sette, L.D., da Mota, A.J., do Nascimento Neto, J.F., Rodrigues, A., de Oliveira, T.B., de Oliveira, F.M., de Oliveira, L.A., dos Santos Barroso, H., and Zanotto, S.P., Filamentous fungi isolates of contaminated sediment in the Amazon region with the potential for benzo(a)pyrene degradation, Water Air Soil. Pollut., 2016, vol. 227, p. 431.
Dramae, A., Intaraudom, C., Bunbamrung, N., Boonyuen, N., Auncharoen, P., and Pittayakhajonwut, P., Antimicrobial tanzawaic acid derivatives from the endophytic Penicillium citrinum BCC71086, Tetrahedron, 2022, vols. 106−107, p. 132645.
Garyali, S., Kumar, A., and Reddy, M.S., Taxol production by an endophytic fungus, Fusarium redolens, isolated from Himalayan yew, J Microbiol Biotechnol., 2013, vol. 23, pp. 1372−1380.
Gautam, V.S., Singh, A., Kumari, P., Nishad, J.H., Kumar, J., Yadav, M., Bharti, R., Prajapati, P., and Kharwar, R.N., Phenolic and flavonoid contents and antioxidant activity of an endophytic fungus Nigrospora sphaerica (EHL2), inhabiting the medicinal plant Euphorbia hirta (dudhi) L., Arch. Microbiol., 2022, vol. 204, p. 140.
Gonelimali, F.D., Lin, J., Miao, W., Xuan, J., Charles, F., Chen, M., and Hatab, S.R., Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms, Front Microbiol., 2018, vol. 9.
Kadaikunnan, S., Rejiniemon, T., Khaled, J.M., Alharbi, N.S., and Mothana, R., In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens, Ann. Clin. Microbiol. Antimicrob., 2015, vol. 14, p. 9.
Keller, N.P., Fungal secondary metabolism: regulation, function and drug discovery, Nat. Rev. Microbiol., 2019, vol. 17, pp. 167−180.
Kim, H., You, Y.H., Yoon, H., Seo, Y., Kim, Y.E., Choo, Y.S., Lee, I.J., Shin, J.H., and Kim, J.G., Culturable fungal endophytes isolated from the roots of coastal plants inhabiting korean East coast, Mycobiology, 2014, vol. 42, pp. 100−108.
Kumar, P., Singh, B., Thakur, V., Thakur, A., Thakur, N., Pandey, D., and Chand, D., Hyper-production of taxol from Aspergillus fumigatus, an endophytic fungus isolated from Taxus sp. of the Northern Himalayan region, Biotechnol. Rep., 2019, vol. 24, p. e00395.
Kumar, S., Stecher, G., and Tamura, K., MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets, Mol. Biol. Evol., 2016, vol. 33, pp. 1870−1874.
Lee, D., Shim, S., and Kang, K., 4,6'-Anhydrooxysporidinone from Fusarium lateritium SSF2 induces autophagic and apoptosis cell death in MCF-7 breast cancer cells, Biomolecules, 2021, vol. 11.
Li, H. and Cui, B., Taxonomy and phylogeny of the genus Megasporoporia and its related genera, Mycologia, 2013, vol. 105, pp. 368−383.
Mohana, K.P., Zuehlke, S., Priti, V., Ramesha, B.T., Shweta, S., Ravikanth, G., Vasudeva, R., Santhoshkumar, T.R., Spiteller, M., and Uma Shaanker, R., Fusarium proliferatum, an endophytic fungus from Dysoxylum binectariferum Hook f., produces rohitukine, a chromane alkaloid possessing anti-cancer activity, A. van Leeuwenjoek, 2012, vol. 101, pp. 323−329.
Ngo, C.C., Nguyen, Q.H., Nguyen, T.H., Quach, N.T., Dudhagara, P., Vu, T.H.N., Le, T.T.X., Le, T.T.H., Do, T.T.H., Nguyen, V.D., Nguyen, N.T., and Phi, Q.-T., Identification of fungal community associated with deterioration of optical observation instruments of museums in Northern Vietnam, Appl. Sci., 2021, vol. 11, p. 5351.
Saithong, P., Panthavee, W., Stonsaovapak, S., and Congfa, L., Isolation and primary identification of endophytic fungi from Cephalotaxus mannii trees, Maejo Int. J. Sci. Technol., 2010, vol. 4, pp. 446−453.
Santos, C., Santos da Silva, B.N., Amorim Ferreira e Ferreira, A.F.T., Santos, C., Lima, N., and Silva Bentes, J.L.d., Fungal endophytic community associated with guarana (Paullinia cupana var. sorbilis): diversity driver by genotypes in the centre of origin, J. Fungi, 2020, vol. 6, p. 123.
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., Warren, J.T., Bokesch, H., Kenney, S., and Boyd, M.R., New colorimetric cytotoxicity assay for anticancer-drug screening, J. Natl. Cancer Inst., 1990, vol. 82, pp. 1107−1112.
Soliman, S.S. and Raizada, M.N.J.F.i.m., Darkness: a crucial factor in fungal taxol production, Front. Microbiol., 2018, vol. 9, p. 353.
Stierle, A., Strobel, G., and Stierle, D., Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew, Science, 1993, vol. 260, pp. 214−216.
Stierle, A.A. and Stierle, D.B., Bioactive compounds from four endophytic Penicillium sp. of a northwest Pacific yew tree, Stu. Nat. Prod. Che., 2000, vol. 1, pp. 933−977.
Tang, Z., Wang, Y., Yang, J., Xiao, Y., Cai, Y., Wan, Y., Chen, H., Yao, H., Shan, Z., Li, C., and Wang, G., Isolation and identification of flavonoid-producing endophytic fungi from medicinal plant Conyza blinii H. Lév that exhibit higher antioxidant and antibacterial activities, PeerJ, 2020, vol. 8, p. e8978.
Toghueo, R.M.K., Bioprospecting endophytic fungi from Fusarium genus as sources of bioactive metabolites, Mycology, 2020, vol. 11, pp. 1−21.
Tsuda, M., Sasaki, M., Mugishima, T., Komatsu, K., Sone, T., Tanaka, M., Mikami, Y., Kobaya-shi, J.I., Scalusamides A−C, new pyrrolidine alkaloids from the marine-derived fungus Penicillium citrinum, J. Nat. Prod., 2005, vol. 68, pp. 273−276.
Vu, T.H.N., Pham, N.S., Le, P.C., Pham, Q.A., Quach, N.T., Do, T.T., Chu, H.H., and Phi, Q.T., Distribution, cytotoxicity, and antioxidant activity of fungal endophytes isolated from Tsuga chinensis (Franch.) Pritz. in Ha Giang province, Vietnam, Ann. Microbiol., 2022, vol. 72, pp. 1−12.
Yang, H.R., Hu, X.P., Jiang, C.J., Qi, J., Wu, Y.C., Li, W., Zeng, Y.J., Li, C.F., and Liu, S.X., Diversity and antimicrobial activity of endophytic fungi isolated from Cephalotaxus hainanensis Li, a well-known medicinal plant in China, Lett. Appl. Microbiol., 2015, vol. 61, pp. 484−490.
Zhang, P., Zhou, P.P., Jiang, C., Yu, H., and Yu, L.J., Screening of taxol-producing fungi based on PCR amplification from Taxus, Biotechnol., Lett., 2008, vol. 30, pp. 2119−2123.
ACKNOWLEDGMENTS
The authors would like to thank the support of VAST– Culture Collection of Microorganisms, Institute of Biotechnology, Vietnam Academy of Science and Technology (www.vccm.vast.vn).
Funding
This study was financially supported by Vietnam Academy of Science and Technology under Grant number TĐCNSH.05/20-22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Supplementary Information
Rights and permissions
About this article
Cite this article
Vu, T.H., Quach, N.T., Le, P.C. et al. Bioprospecting Endophytic Fungi Isolated from Cephalotaxus mannii Hook f. as Prolific Sources of Antibacterial, Anticancer, and Antioxidant Agents. Microbiology 92, 284–292 (2023). https://doi.org/10.1134/S0026261722602834
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722602834