Abstract
Twenty-eight endophytic bacteria isolated from stems and leaves of healthy plants in Kanchanaburi Province, Thailand, were characterized and evaluated for their plant growth-promoting activity. Based on their phenotypic characteristics and 16S rRNA gene sequence similarity, the isolates were identified as Pantoea (6 isolates), Priestia (4 isolates), Pseudomonas (3 isolates), Enterobacter (2 isolates), Acinetobacter (2 isolates), Novosphingobium (2 isolates), Curtobacterium (2 isolates), as well as Bacillus, Peribacillus, Sphingobium, Staphylococcus, Brevibacillus, Aneurinibacillus, and Pseudarthrobacter (1 isolate each). Seven isolates produced indole-3-acetic acid (IAA) in nitrogen-free broth supplemented with 0.01% L-tryptophan, eleven isolates fixed nitrogen, and twenty-three isolates solubilized phosphate and zinc. Sphingobium sp. Sx8-8 produced the highest IAA at 67.29 µg mL–1, followed by Novosphingobium sp. SI8-3 and Pantoea sp. S5-1 at 33.06 and 29.4 µg mL–1, respectively. The maximum IAA produced by the Sphingobium sp. Sx8-8 increased to 232.1 µg mL–1 at optimized conditions. In in vitro rice germination, the Sphingobium sp. Sx8-8 and Pantoea sp. S5-1 increased root length, number of lateral roots, and shoot length more than uninoculated control and synthetic IAA as a standard. The genome size of Sphingobium sp. Sx8-8 was 4.67 Mb (83 contigs), N50 size and average G + C content were 150 389 bp and 64.2 mol %, respectively. This study indicated that endophytic bacteria could potentially promote plant growth and be utilized as bioinoculant or biofertilizers in agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endophytic bacteria colonize intra- and intercellular plant tissues throughout their life cycle without causing plant disease. They stimulate plant growth by (1) fixing nitrogen from the air and converting it to ammonia which is utilized by plants (Kirchhorf et al., 1997); (2) producing siderophores (under soil Fe-deficiency conditions) which form complexes with Fe ions, leading to an increase in plant Fe-uptake; (3) producing plant hormones, including indole-3-acetic acid (IAA), which is involved in cell development and plant root elongation (Normanly et al., 1995); and (4) solubilizing insoluble phosphorus (P), zinc (Zn), and potassium (K) in soil which increases availability of P, Zn and K to plants (Meena et al., 2016). Endophytic bacteria are found in most healthy plants; however, the structure of endophytic bacterial communities is related to plant physiology which is influenced by various factors (i.e., environmental variability and climate change) (Hutchinson, 1961; Chesson and Warner, 1981), including temperature, humidity, light, and nutrients. Endophytic bacteria of the genera Rhizobium, Pseudomonas, Bacillus, Azospirillum, Sphingomonas, Burkholderia, Enterobacter, Paenibacillus, Nocardia, Streptomyces, and Microbacterium occurred mainly in cultivated plants (Rosenblueth and Martínez-Romero, 2006).
Several researches have focused on endophytic bacterial population, diversity, and capability to promote plant growth. Nevertheless, there are few reports on the endophytic bacterial population in the forests of Southeast Asia. The goal of the present work was to isolate endophytic bacteria from stems and leaves of various plants in Kanchanaburi Province, Thailand, and to identify them based on phenotypic and genotypic characteristics. The genomic data of a selected plant-promoting isolate was analyzed for genome attributes, IAA production, and phosphate, and zinc solubilization. In addition, IAA production by the selected isolate was optimized, and the effect of IAA-producing bacteria on in vitro rice germination was investigated.
MATERIALS AND METHODS
Sources and isolation methods. Stems and leaves of eight plant samples, including Thyrsostachys siamensis, Swietenia mahagoni, and Bambusa multiplex, were collected from the nearby Erawan National Park, while Afzelia xylocarpa, Toona ciliata, Crateva religiosa, Phyllanthus emblica, and Kaempferia marginata were from Si Sawat district, in Kanchanaburi Province, Thailand. The plant samples were stored in plastic bags and transported to the laboratory for plating within 24 h. The stems and leaves were washed with sterile water and then cut into 6-cm long pieces with a sterile knife. Surface sterilization of stems and leaves was performed by subsequent soaking in a series of solutions as follows: 2% sodium hypochlorite for 3 min, sterile distilled water for 3 min, 70% ethanol for 1 min, and finally washing three times in sterile distilled water. The sterilized stems and leaves were cut into small segments with a sterile knife, spread on nutrient agar (NA) that was diluted threefold by water, and incubated at 30°C for 24–48 h. A single bacterial colony was picked up and purified on NA for further study.
Identification Methods
Phenotypic characteristics. Bacterial isolates were identified based on phenotypic characteristics, including colony and cell morphology, Gram stain, catalase and oxidase activity, hydrolysis of starch, lipid, gelatin, casein, arginine, and esculin (Sierra, 1957). Effects of temperature (40 and 45°C), pH (5, 6, 8, 9) and NaCl concentration (1, 3, and 5%) on growth of the isolates were tested on NA agar at 30°C for 1–3 days. The formation of acid from different sugars was determined as previously described (Tanasupawat et al., 1998).
Genotypic characteristics. Repetitive sequence-based polymerase chain reaction (Rep-PCR) using (GTG)5 primer (5'-GTGGTGGTGGTGGTG-3') targeted against conserved repetitive sequences was selected for strain typing, and the PCR reactions were carried out according to Versalovic et al. (1994) and Tolieng et al. (2018). Amplification was done in a 25 µL reaction mixture consisting of approximately 50 ng µL–1 DNA, 10× PCR reaction buffer containing 20 mM MgCl2, 20 pmol µL–1 (GTG)5 primer, 2.5 mM dNTPs mixture, and 2 U µL–1 Takara Taq DNA polymerase (Takara Bio Inc., Japan). The thermocycling program was denaturation at 95°C for 5 min, followed by 30 cycles each of denaturation at 94°C for 45 s, annealing at 40°C for 60 s, and primer extension at 65°C for 10 min, followed by a final elongation at 65°C for 20 min using the Bio-Rad T100 PCR thermal cycler (Bio-Rad Laboratories, United States). PCR products (10 µL) were analyzed by electrophoresis using 1% agarose gel (15 × 25 cm) with a constant voltage of 150 V in 0.5× Tris-boric acid-EDTA acid (TBE) buffer for 2.20 h at 25°C. The gel was stained in 0.5 µg mL-1 ethidium bromide solution, and the 100 to 10 000 bp GeneRuler DNA Ladder Mix (Thermo Fisher Scientific Baltics UAB) was used as a molecular size marker. The gel was visualized under a UV transilluminator using the Gel document™ XR+ imaging system (Bio-Rad, United States). Genetic relationships among the endophytic bacterial isolates were analyzed from the resultant fingerprints using the software package GelCompar II version 5.10 (Applied Maths, Belgium). The dendrogram for all isolates was generated by cluster analysis using the unweighted pair group method with average linkages (UPGMA) clustering algorithm. A clustering level of 80% was regarded as a significant grouping (Gevers et al., 2001; Tolieng et al., 2018).
The 16S rRNA gene was amplified by PCR using universal primers 20F (5'-AGAGTTTGATCATGGCTCAG-3'), and 1500 R (5'-GGTTACCTTGTTACGACTT-3') and the amplified 16S rRNA gene sequence was analyzed by Macrogen® Korea. Universal primer 800R (5'-TACCAGGGTATCTAATCC-3'; 802-785) was used for sequencing the partial 16S rRNA regions and 4 universal primers, 800R, 27F (5'‑AGAGTTTGATCCTGGCTCAG-3'; 8-27), 518F (5'-CCAGCAGCCGCGGTAAT-3'; 542-518), and 1492R (5'-GGTTACCTTGTTACGACTT-3'; 1492-1507) were used for full-length sequencing of the 16S rRNA gene. The sequences of the isolates were aligned with selected sequences from GenBank using CLUSTAL_X version 1.81. The alignment was manually edited to remove gaps, and ambiguous nucleotides before phylogenetic trees were constructed using maximum-likelihood (ML) (Felsenstein, 1981) with the program MEGA11 (Koichiro et al., 2021). Confidence values for individual branch in the phylogenetic tree were determined using bootstrap analysis (Felsenstein, 1985) based on 1000 replicates. The sequence similarity values between the isolates and their related neighbors were calculated using the EzBiocloud service (Yoon et al., 2017).
Determination of Plant Growth-Promoting Activity
Phosphate (P) and zinc (Zn) solubilization. Pikovskaya’s agar containing 0.5% Ca3(PO4)2 and mineral salt agar containing 0.1% ZnO were used to examine of P and Zn solubilization, respectively. The bacterial isolates grown for 24 h as lawns on nitrogen-free (NF) agar containing the following (g L–1): glucose, 10; K2HPO4, 1; MgSO4, 0.2; CaCO3, 1; NaCl, 0.2; Na2MoO4·2H2O, 0.005; FeSO4, 0.1; and agar, 15. Agar cylinders (6 mm diameter) were then cut using a sterile cork borer, placed on the indicator media and incubated at 30°C for 7 days and 1 day, respectively. The halo zone around the agar indicated the solubilization activity. Solubilization index (SI) was calculated from a ratio of the halo zone and agar diameter.
Nitrogen-fixing activity. All bacterial isolates were tested for their ability to fix nitrogen using the Nessler’s reagent method (Svehla, 1979) by cultivation in NF broth at 30°C for 48 h, centrifugation at 1107 g for 10 min, and mixing 3 mL of the obtained supernatant with 60 µL Nessler’s reagent. Development of yellow-orange color indicated ammonia synthesis. Nitrogen-fixation activity was determined from absorbance of the developed yellow-orange color at 560 nm against uninoculated NF broth.
Indole-3-acetic acid (IAA) production. Indole-3-acetic acid (IAA) production was determined colorimetrically using the Salkowski’s reagent (49 mL of 35% H2SO4 : 1 mL of 0.5 M FeCl3). For qualitative measurement of IAA, the bacterial isolates were cultured individually (108 CFU mL–1) in NF broth containing 0.01% L-tryptophan and incubated for 24 h at 30°C with shaking (150 rpm). After centrifugation at 7871 g for 20 min, 70 µL of the supernatant was mixed with 140 µL Salkowski’s reagent and incubated at room temperature for 20 min in the dark. The development of pink color indicated IAA production. The absorbance at 530 nm of the developed pink color was measured and compared with the standard curve for IAA. NF broth containing tryptophan mixed with Salkowski’s reagent was used as the control.
Production of IAA by isolate Sx8-8 was confirmed by the modified method of Bhutani et al. (2018). Briefly, the isolate was cultured in 100 mL NF broth supplemented with 0.01% L-tryptophane at 30°C, 200 rpm in the dark for 48 h. After centrifugation at 7871 g for 20 min, the supernatant was adjusted to pH 2.5–3 with 1 N HCl and extracted twice with an equal volume of ethyl acetate. After removal of residual ethyl acetate from the resultant crude extract on a vacuum rotary evaporator at 40°C, the crude extract was dissolved in 2 mL HPLC grade methanol. Analysis of IAA was done by reversed-phase HPLC 1260 (Agilent, United States) using a C18 column with the diode-array detector at 280 nm. The mobile phase was deionized water: methanol (55 : 45) at a 1 mL min−1 flow rate. Peak-retention time compared to synthetic IAA.
Optimization of IAA production. Cultivation conditions for IAA production of isolate Sx8-8 and S5-1 in NF broth supplemented with 0.01% L-tryptophan (pH 6.8) at 30°C, 150 rpm shaking for 24 h were optimized by varying pH (4, 5, 6, 7, and 8), temperature (25, 30, 35, and 40°C), L-tryptophan concentration (0.5, 1, 1.5, and 2%) and incubation period (24, 48, 72, 96, and 120 h); one factor at a time (univariate analysis). Three independent repeats were performed per treatment. The condition that gave the highest IAA production was then selected for the subsequent experiments.
Effects of IAA-producing isolates on rice germination. IAA-producing endophytic bacteria, isolate Sx8‑8 and S5-1, were examined for their ability to promote in vitro rice germination. Cultures of isolate Sx8-8 and S5-1 (108 CFU mL–1) were prepared by growing in NF broth supplemented with 0.01% L-tryptophan at 30°C, 150 rpm shaking for 24 h. Seeds of glutinous rice variety Rice Department 6 (RD6) were surface sterilized by soaking in 5% sodium hypochlorite for 15 min and then rinsed five times with sterile water (Zhang et al., 2019). The sterilized rice seeds were soaked in the prepared cultures of isolate Sx8-8 and S5-1; various synthetic IAA concentrations (10, 30, and 50 µg mL–1), or sterile distilled water for 3 h (Yu et al., 2016) and then germinated on Murashige and Skoog (MS) basal medium at 25°C in the dark for 15 days. Ten independent repeats were performed per treatment. Differentiation of rice seedlings was observed daily. Root length, shoot length, number of lateral roots, root fresh weight, shoot fresh weight, root dry weight, and shoot dry weight were recorded.
Statistical analysis. The results were reported as means values ± standard deviation (SD). Analysis of Variance (ANOVA) and Duncan’s Multiple Range Test (DMRT) were used to analyze and compare differences between treatments, and p values ≤0.05 were considered to be statistically significant.
Genome analysis of isolate Sx8-8. Genomic DNA of isolate Sx8-8 was extracted using the GenepHlowTM Gel/PCR kit (Geneaid). The quality of the extracted DNA was examined using a NanoDrop 2000c spectrophotometer, Thermo Fisher Scientific Inc., United States and agarose gel electrophoresis. Genome sequencing was carried out using an Illumina MiSeq sequencer at Omics Sciences & Bioinformatics Center, Chulalongkorn University, Thailand. The genome was assembled using Unicycler, and the genome was annotated using the software Prokka version 1.13. Rapid Annotation using Subsystem Technology (RAST) server (http://rast.nmpdr.org/) (Aziz et al., 2008) and SEED Viewer (Overbeek et al., 2014) were used for investigation of metabolic features, and functional genes. Comparative analyzes between genome of isolate Sx8-8 and its closely related type strains were estimated based on average nucleotide identity-BLAST (ANIb), average amino acid identity (AAI) and digital DNA-DNA hybridization (dDDH). The values of ANI and AAI were calculated by using microbial genomes atlas (MiGA) web service (http://microbial-genomes.org). The dDDH was calculated by Genome-to-Genome Distance Calculator (GGDC 2.1) using the BLAST+ method.
RESULTS AND DISCUSSION
Identification of Isolates
Twenty-eight bacterial isolates were isolated from stems and leaves of the plant samples, which were collected from the Erawan National Park (11 isolates) and Si Sawat district (17 isolates). Phenotypic characteristics and 16S rRNA gene sequence similarity were used for preliminary species identification of the isolates (Table 1). Seventeen gram-negative and 11 gram-positive isolates were catalase-positive, rods-shaped bacteria (except isolate A1-1). They grew in 1% NaCl at pH 6 but could not hydrolyze lipid. Based on their 16S rRNA gene sequence similarity, they were divided into 14 groups (Tables 1 and 2).
Group A contained 4 isolates of spore-forming bacteria, A2-2, A2-3, A2-8, and A2-5. They grew in 3% NaCl at 40 and 45°C and hydrolyzed starch. Isolate A2-2, A2-3, and A2-8 hydrolyzed casein, gelatin, and esculin, whereas isolate A2-5 hydrolyzed only arginine. They produced acid from several sugars. Isolate A2-2, A2-3, and A2-8 were closely related to Priestia aryabhattai B8W22T (100%), whereas isolate A2-5 was closely related to Priestia megaterium NBRC 15308T (100% similarity).
Group B contained 1 isolate, A3-1. This isolate grew in 3% NaCl, at pH 5, at 40 and 45°C, and hydrolyzed starch, casein, gelatin, and esculin, but could not produce acid from any sugars. Isolate A3-1 was closely related to the taxonomic group containing Brevibacillus parabrevis NRRL NRS 605T, B. schisleri ATCC 35690T, B. brevis NBRC 15304T, B. choshinensis DSM 8552T, and B. reuszeri DSM 9887T (98.01–99.86% similarity).
Group C contained 1 isolate, SI8-4. This isolate grew in 3 and 5% NaCl, at pH 5 and 8, at 40 and 45°C; it produced acid only from raffinose but could not hydrolyze starch, casein, gelatin, esculin and L-arginine. Isolate SI8-4 was closely related to the taxonomic group including Peribacillus butanolivorans DSM 18926 T, P. muralis DSM 16288T, and P. simplex NBRC 15720T (98.37–98.64% similarity).
Group D contained 2 isolates, S6-1 and S6-3. They grew in 3, 5% NaCl, and at pH 5. They hydrolyzed only esculin and produced acid from various sugars. Isolate S6-1 and S6-3 were closely related to the taxonomic group including Curtobacterium citreum DSM 20528T, C. oceanosedimentum ATCC 31317T, C. albi-dum DSM 20512T, C. flaccumfaciens LMG 3645T, and C. luteum DSM 20542T (99.22–99.85% similarity).
Group E contained 1 isolate, A1-1. This isolate grew in 3 and 5% NaCl, at pH 5, 8 and 9, at 40°C, and produced acid from various sugars, while only gelatin was hydrolyzed. Isolate A1-1 was closely related to taxonomic group including Staphylococcus hominis subsp. hominis DSM 20328T, S. hominis subsp. novobiosepticus GTC 1228T, S. borealis 51-48T, and S. haemolyticus MTCC 3383T (99.02–99.86% similarity).
Group F contained 6 isolates, A1-2, S5-1, S5-3, Sx8-4, Sx8-6, and Sx8-7. They could grow in 3 and 5% NaCl, at pH 5, 8, and 9, and at 40 and 45°C, hydrolyzed esculin, and produced acid from various sugars except for raffinose and xylose. All isolates were closely related to Pantoea dispersa LMG 2603T (100% similarity).
Group G contained 2 isolates, S7-2 and S7-4. They grew in 3 and 5% NaCl, at pH 5 and 8, at 40 and 45°C, hydrolyzed only arginine and produced acid from various sugars. Isolate S7-2 and S7-4 were closely related to Acinetobacter baumannii ATCC 19606T (100% similarity).
Group H contained 2 isolates, S4-1 and S5-2. They grew in 3 and 5% NaCl, at pH 8 and 9, at 40°C, and hydrolyzed only arginine. Isolate S4-1 and S5-2 could produce acid from several sugars but not from lactose, raffinose, and sorbitol. Two isolates were closely related to Enterobacter hormaechei subsp. xiangfangensis LMG 27195T (100% similarity).
Group I contained 3 isolates, A2-4, A2-6, and A2‑9, which could grow in 3 and 5% NaCl, at pH 5, 8, and 9, but could not grow at 40 and 45°C. They hydrolyzed only arginine and produced acid from galactose, sucrose, fructose, and xylose. All isolates were closely related to the taxonomic group including Pseudomonas entomophila L48T, P. asiatica RYU5T, P. taiwanensis BCRC 17751T, P. monteilii NBRC 103158T, P. inefficax JV551A3T, P. mosselii CIP 105259T, and P. plecoglossicida NBRC 103162T (99.29–99.59% similarity).
Group J contained 1 isolate, A2-10. This isolate grew at pH 5, 8 and 9, at 40°C, hydrolyzed arginine, and produced acid from sucrose, fructose lactose, ribose, and sorbitol. Isolate A2-10 was closely related to the taxonomic group including Aneurinibacillus aneurinilyticus ATCC 12856T (99.79% similarity) and A. migulanus DSM 2895T (99.10% similarity).
Group K contained 1 isolate, S7-6. This isolate grew at pH 5, 8 and 9, at 40°C, hydrolyzed arginine, starch, gelatin, and produced acid from sucrose, fructose, galactose, and xylose. Isolate S7-6 was closely related to Bacillus stercoris JCM 30051T (100% similarity).
Group L contained 2 isolates, SI8-2 and Sx8-5. They grew at pH 5 and 8, hydrolyzed esculin, and produced acid from arabinose, galactose, and maltose. Isolate SI8-2 produced acid from lactose, cellobiose, and fructose, while Sx8-5 produced acid from xylose. Isolate SI8-2 and Sx8-5 were closely related to the taxonomic group including Novosphigobium clariflavum 164T, N. barchaimii LL02T, N. naphthalenivorans NBRC 10205T, N. resinovorum NCIMB 8767T, AKFJ_s, N. panipatense SM16T, N. mathurense SM117T, N. gossypii JM-1396T, CP030353_s, N. lindaniclasticum LE124T, and N. silvae FGD1T (98.40–99.85% similarity).
Group M contained 1 isolate, SI8-5. This isolate grew at pH 5 and 8, hydrolyzed only esculin, and produced acid from arabinose and maltose. Isolate SI8-5 was closely related to the taxonomic group including Pseudarthrobacter phenanthrenivorans SWC37T, P. enclensis NIO-1008T, P. phenanthrenivorans Sphe3T, P. defluvii 4C1-aT, P. niigatensis LC4T, P. siccitolerans 4J27T, P. chlorophenolicus A6T, and P. equi IMMIB L-1606T (98.05–99.86% similarity).
Group N contained 1 isolate, Sx8-8. This isolate grew at pH 5 and 8, hydrolyzed only esculin, and produced acid from arabinose, maltose, galactose, and xylose. Isolate Sx8-8 was closely related to the taxonomic group including Sphingobium chungbukens DJ77T, S. estronivorans AXBT, S. indicum B90AT, S. aromaticivastans UCM-25T, S. chlorophenolicum NBRC 16172T, and S. herbicidovorans NBRC 16415T (97.09–98.59% similarity).
The (GTG)5-PCR analysis was done for grouping of the 28 isolates, and the dendrogram of rep-PCR fingerprints as shown in Fig. 1. The DNA fragments of sizes ranging from 300 to 8000 bp were generated and yielded 6−25 bands and displayed 0.5–99.5% similarity. After cluster analysis, 17 groups were defined. The most numerous of the isolates (21.4%) contained 6 isolates (A1-2, S5-1, S5-3, Sx8-4, Sx8-6, and Sx8-7) and were closely related to P. dispersa, while the rest of the isolates were distributed in other groups. Most bacterial fingerprinting was classified into several groups, but the rest of the isolates did not form clusters based on phenotypic characteristics. This result confirmed that rep-PCR is a powerful molecular tool suitable for grouping, identifying, and differentiating bacterial strains of the same species (Gomez-Gil et al., 2004). Moreover, the (GTG)5-PCR fingerprinting increases our knowledge of bacterial biodiversity in the environment.
Based on the partial 16S rRNA gene sequence analysis, the 28 isolates were identified as members of 14 genera. These diverse bacteria were widely distributed in Kaempferia marginata followed by Swietenia mahagoni. However, since many isolates shared the similarity to the related type strain below or close to the cut-off value of 98.7% (Stackebrand and Ebers, 2006), belonging probably to novel species, further studies are required.
Determination of Plant Growth-Promoting Activity
Phosphate (P) and zinc (Zn) solubilization. Phosphorus (P) in soil forms insoluble complexes with organic compounds which are unavailable to plants. Phosphate-solubilizing bacteria (PSB) increase phosphorus availability to plants by secreting organic acids and phosphatases to convert the insoluble phosphate complex into soluble monobasic (\({{{\text{H}}}_{{\text{2}}}}{\text{PO}}_{4}^{ - }\)) and dibasic (\({\text{HPO}}_{4}^{{ - 2}}\)) ions (Ramanuj and Shelat, 2018). In the present study, the P solubilization capacity of isolates was assessed using Ca3(PO4)2; most isolates (82%) exhibited an ability to solubilize Ca3(PO4)2. Isolate Sx8-5 (N. barchaimii) gave the highest SI at 2.5 (Table 3), which agreed well with Song et al. (2022), who reported that N. resinovorum Y5 showed a zone of Ca3(PO4)2 solubilization. However, culture media and phosphate compounds used as a P source are known to affect the screening of PSB (Oliveira et al., 2009).
Zinc (Zn) is an essential micronutrient required in a small amount for living organisms. Zn deficiency in the soil leads to retarded flower and fruit development, and a decrease in the synthesis of phytohormones and carbohydrates (Welch and Graham, 2004). Applied chemical fertilizers such as Zn-sulfates, Zn-ammonia complexes, Zn-nitrate, Zn-oxide, Zn-oxysulfate, Zn-carbonate, and Zn-chloride have been found to remain on the soil surface and unavailable to plants (Khanghahi et al., 2018). In this study, twenty-three isolates could solubilize zinc oxide in a mineral salt agar medium. Isolate Sl8-5 (P. phenanthrenivorans) showed the highest SI at 6, followed by isolate A2-4, A2-6, A2-9 (P. entomophila), S4-1 (E. hormaechei subsp. xiangfangensis), and SI8-4 (P. butanolivorans) at a range of 3.4–4.6 (Table 3). The results indicated that most of the isolates were zinc-solubilizing bacteria (ZSB) which increased zinc availability to plants. These isolates might solubilize Zn through the production of acids, and siderophores (Alexander, 1997; Saravanan et al., 2007).
Dominant bacteria isolated in this study belonged to the genera Pantoea, Priestia, and Pseudomonas, which occur in many ecological environments. These bacteria could solubilize both P and Zn, which has been reported by several previous studies (Kumari et al., 2018; Khamwan et al., 2018; Ramanuj and Shelat, 2018). The amount of the P and Zn solubilized by bacteria depends on strain efficiency and environment.
Nitrogen-fixing activity. Twenty-eight isolates were evaluated for their ability to fix nitrogen using the Nessler’s reagent method. Eleven isolates were able to fix nitrogen (Table 3). Nitrogen is a crucial limiting factor for plant growth because it is an essential component of all amino acid in plant structure, enzymes and nucleic acids that form DNA, and enhance chlorophyll in plants. Plants cannot utilize atmospheric nitrogen for growth and metabolism; thus, nitrogen must be reduced to ammonia by nitrogen-fixing bacteria.
IAA production. Among twenty-eight isolates screened, seven isolates (A1-2, S5-1, S5-2, S6-3, SI8‑5, Sx8-6, and Sx8-8) produced IAA at concentrations ranging from 6.13 to 67.29 µg mL–1 (Fig. 2a). Tryptophan served as the primary precursor for IAA biosynthesis in bacteria via the Trp-dependent pathway (Spaepen et al., 2007). Sphingobium sp. Sx8-8 produced the highest IAA amount (67.29 µg mL–1). Similar results were reported by Rodrigues et al. (2018). They found that S. yanoikuyae BU32 gave the highest IAA compared with Burkholderia, Sphingobium, Rhizobium, and Enterobacter strains isolated from the rhizospheric soil of sugarcane. Production of IAA by Sphingobium sp. Sx8-8 was confirmed by HPLC analysis as a peak was observed at 2.54 min retention time, which corresponded to the peak of synthetic IAA standard at 2.56 min (Fig. 2b).
Indole-3-acetic acid (IAA) production of isolates cultivated in NF broth containing 0.01% L-tryptophan (a). The data represent the average of independent experiments (n = 3). Different alphabet letters indicate significant difference between treatments using Duncan’s multiple range test (p ≤ 0.05), HPLC analysis of IAA production from crude extract of isolate Sx8-8 and standard IAA using water and methanol as mobile phase, overlay peak IAA of isolate Sx8-8 and standard IAA (b).
Optimization of IAA production. The isolate Sphingobium sp. Sx8-8 and Pantoea sp. S5-1 were able to produce IAA at pH 6 and 7, but highly acidic (pH 5) and strongly alkaline (pH 8) were considered unsuitable for IAA production. Maximum amounts of IAA were produced by the isolate Sx8-8 (76.19 µg mL–1) and S5-1 (21.9 µg mL–1) at pH 7 (Fig. 3a). The pH is a crucial parameter for microbial growth. Kumari et al. (2018) reported that pH 7 was optimal for IAA production by B. subtilis DR2 (158.79 µg mL–1). Apine and Jadhav (2011) reported that P. agglomerans strain PVM showed maximum IAA (1.441 g L–1) at pH 7, similar to maximum IAA production found at pH 7.5 for Pseudomonas putida UB1, which has been demonstrated by Bharucha and Patel (2013). However, pH 6 has also been reported as optimal for Acetobacter diazotrophicus L1 isolated from sugarcane (Patil et al., 2011). Both isolates could produce IAA at 25, 30, 35, and 40oC; nevertheless, the production of IAA was affected by high temperature, as shown in Fig. 3b. Sphingobium sp. Sx8-8 and Pantoea sp. S5-1 produced the highest IAA at 30 and 25°C, respectively. The maximum IAA produced by Sphingobium sp. Sx8-8 was 35.41% increased when the concentration of L‑tryptophan in NF broth was increased from 0.01 to 0.5%. A decrease in IAA production was observed at a high concentration of tryptophan (Fig. 3c), whereas the maximum IAA production of Pantoea sp. S5-1 was achieved at 1.5% L-tryptophan. Moreover, 2% L‑tryptophan significantly negatively affected the IAA production of both strains. Different bacterial species have different pathways for IAA synthesis, such as the indole-3-pyruvic acid (IPA) pathway, which was found in non-pathogenic plant-associated bacteria, while the indole-3-acetamide (IAM) pathway was found in phytopathogenic bacteria (Duca et al., 2014; Shaik et al., 2016). Our results indicated that IAA synthesis affected by tryptophan utilization differed in different species. Production of IAA by Sphingobium sp. Sx8-8 was highest (232.1 µg mL–1) in NF broth supplemented with 0.5% L-tryptophan (pH 7) at 30°C after 48 h of incubation, then slightly decreased as shown in Fig. 3d. At 48 h, Sphingobium entered the stationary phase of growth (Flood et al., 2018), and IAA is a secondary metabolite. This result might be a reason why Sphingobium sp. Sx8-8 produced maximum IAA at 48 h. Rodrigues and Forzani (2016) have demonstrated that S. yanoikuyae BU32 cultivated in TSB supplemented with 5 mM L-tryptophan for 72, 96, and 120 h gave IAA higher than those cultivated for 24 h. Based on the results obtained, IAA production was affected by pH, temperature, L-tryptophan concentration, and incubation time, as well as by the species, and growth phase.
Optimization of IAA production (µg mL–1) of isolates based on different pH (a), temperature (b), L-tryptophan (c), incubation time (d), cultivated in NF medium supplemented with L-tryptophan for 24 h. ◼; Isolate Sx8-8 and  ; Isolate S5-1. The data represent the average of independent experiments, each with replicates ± standard deviation (SD). Different alphabet letters indicate significant difference between treatments using Duncan’s multiple range test (p ≤ 0.05).
; Isolate S5-1. The data represent the average of independent experiments, each with replicates ± standard deviation (SD). Different alphabet letters indicate significant difference between treatments using Duncan’s multiple range test (p ≤ 0.05).
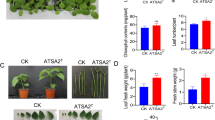
Effects of IAA-producing isolates on rice germination. After 15 days of cultivation, a significant increase in root length and number of lateral roots was observed in rice seeds treated with two IAA-producing isolates, Sphingobium sp. Sx8-8 and Pantoea sp. S5-1, as well as with synthetic IAA. The isolate Sphingobium sp. Sx8-8 increased root length by 80% compared to the control and by 23% compared to 50 µg mL–1 synthetic IAA. The effect of Pantoea sp. S5-1 on root length was lower than those of Sphingobium sp. Sx8-8 and synthetic IAA, but 20.9% higher than the control (Table 4). Standard IAA at 10 µg mL–1 gave the highest root length. An increase in synthetic IAA concentration to 30 and 50 µg mL−1 decreased root length (Fig. 4a) which coincided with the results of Kukavica et al. (2007). High IAA concentration has an inhibitory effect on root elongation due to induction of the synthesis of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase for ethylene formation. A low concentration of IAA inhibits ethylene formation and transport of its precursors in plants (Hansen and Grossmann, 2000; Wei et al., 2000). Treatment with Sphingobium sp. Sx8-8 resulted also in the highest shoot length (18.7 cm) (Fig. 4b) and a high number of lateral roots similar to synthetic IAA. The values of all parameters, namely, root and shoot lengths, number of lateral roots, root and shoot fresh weights, and root and shoot dry weights of rice seedlings treated by Pantoea sp. S5-1 were higher than the control. These results indicated that Sphingobium sp. Sx8-8 and Pantoea sp. S5-1, the IAA-producing bacteria, had a positive effect on rice germination. Plant growth-promoting ability of bacteria was reported in several studies. Kalaiselvi and Priya (2020) reported that S. yanoikuyae MH394206 and mixed consortia enhanced plant height and root volume of rice CO51 in moisture deficiency conditions. Makar et al. (2021) reported 12 bacterial strains isolated from wheat grains and belonging to the genera Staphylococcus, Pantoea, Sphingobium, Bacillus, Kosakonia, and Micrococcus to be capable of synthesizing indole-related compounds (IRCs), which are generally considered as IAA precursors. Pantoea has been found in many species of plants, such as sugarcane, olive knots, and the Cactaceae family, and could potentially promote plant growth (Dastager et al., 2009; Rodrigues and Forzani, 2016; Luziatelli et al., 2020). In this study, Sphingobium sp. Sx8-8 and Pantoea sp. S5-1, isolated from the stem of Kaempferia marginata and Toona ciliata, respectively, revealed the capability to enhance rice germination. This result supported the previous reports that endophytic bacteria isolated from one kind of plant could promote growth in another kind of plant. Giassi et al. (2016) reported that IAA-producing Bacillus spp. (strain BM05, BM16, and MB17) isolated from strawberry leaves and Bacillus sp. strain CPM04 isolated from coffee leaves were able to promote the growth of citrus rootstocks. In contrast, IAA-producing endophytic bacteria, Microbacterium sp. C4, and Lysinibacillus sp.C7 isolated from corn roots showed a potential to promote the growth of both soybean and wheat seedlings (Yu et al., 2016). Moreover, endophytic bacteria isolated from plant stem was found to be able to enhance rice root germination. Khamwan et al. (2018) reported that bacteria isolated from the stem and leaves of the Jerusalem artichoke had growth-promoting activity in Jerusalem artichoke tuber, which implied that the endophytic bacteria might spread out to all parts of the plant and function in different systems. Endophytic bacteria colonizing plant roots have beneficial effects on plants differently depending on the bacterial property, plant species, and interaction between the bacteria and plants.
Genomic features of Sphingobium sp. Sx8-8. Isolate Sx8-8 had a genome size of 4.67 Mb with 83 contigs, N50 size of 150 389 bp, an average G+C content of 64.2 mol %, protein-coding sequences (CDS) of 4498, tRNA genes of 51, and rRNA genes of 3. The whole-genome sequence had been deposited in GenBank under the accession number of JAKUJY000000000. Since isolate Sx8-8 was considered as an IAA-producing bacterium, PSB and ZSB (Fig. 2 and Table 3), and boosted the rice growth (Fig. 4 and Table 4), we concentrated on examining the genes that contribute to the potential of this strain. This study found that the genome of this strain contained 4 genes related to auxin biosynthesis with key enzymes that utilize tryptophan and biosynthesis of IAA (data not shown). Based on the 16S rRNA gene sequence (Table 1), isolate Sx8-8 was belonged to the genus Sphingobium and was closely related to Sphingobium chungbukens DJ77T (98.59% similarity). Comparing whole-genome sequences, based on ANI, AAI (accessed 2022-08-18), and dDDH values, ANI obtained between isolate Sx8-8 and Sphingobium japonicum UT26ST (=Sphingobium indicum) (86.46%), Sphingobium sp. TKS (86.32%), Sphingobium chlorophenolicum L-1 (86.17%) and Sphingobium sp. RSMS (86.07%); and AAI obtained between isolate Sx8-8 and Sphingobium japonicum UT26ST (84.59%), Sphingobium sp. RSMS (85.43%), Sphingobium chlorophenolicum L-1 (84.22%) and Sphingobium sp. TKS (83.65%) were below the cutoff at 94–96% (Konstantinidis and Tiedje, 2005; Richter and Rossello-Mora, 2009; Varghese et al., 2015). In addition, the dDDH values between isolate Sx8-8 and related type strains were 29.2–27.9% (data not shown), which were also below the 70% threshold (Auch et al., 2010). Therefore, isolate Sx8-8 was a novel species in the genus Sphingobium; thus, further characterization should be carried out.
CONCLUSIONS
Twenty-eight endophytic bacteria were isolated from stems and leaves of several plants in Kanchanaburi Province, Thailand. Based on phenotypic data and 16S rRNA gene sequence analysis, they were belonged to 14 genera, including Pantoea, Priestia, Pseudomonas, Enterobacter, Acinetobacter, Novosphingobium, Curtobacterium, Bacillus, Peribacillus, Sphingobium, Staphylococcus, Brevibacillus, Aneurinibacillus, and Pseudarthrobacter. Isolates Sx8-5, and Sl8-5 showed the highest P and Zn solubilization, respectively. Sphingobium sp. Sx8-8 was considered as the best strain to produce the highest IAA concentration (232.1 µg mL–1) in the medium supplemented with 0.5% L-tryptophan, pH 7.0, 30°C for 48 h. Additionally, isolate Sx8-8 could also solubilize P and Zn. This study indicated that Sphingobium sp. Sx8-8 had main properties for use as plant growth-promoting bacteria (PGPB) in rice seedlings.
REFERENCES
Apine, O.A. and Jadhav, J.P., Optimization of medium for indole-3-acetic acid production using Pantoea agglomerans strain PVM, J. Appl. Microbiol., 2011, vol. 110, pp. 1235−1244.
Alexander, M., Introduction to Soil Microbiology, New York: Wiley, 1997.
Auch, A.F., von Jan, M., Klenk, H.-P., and Göker, M., Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison, Stand. Genom. Sci., 2010, vol. 2, pp. 117−134.
Aziz, R.K., Bartels, D., Best, A.A., DeJongh, M., Disz, T, Edwards, R.A., Formsma, K., Gerdes, S., Glass, E.M., Kubal, M., Meyer, F., Olsen, G.J., Olson, R., Osterman, A.L., Overbeek, R.A., et al., The RAST server: rapid annotations using subsystems technology, BMC Genomics., 2008, vol. 9, p. 75.
Bharucha, U. and Patel, K., Optimization of indole acetic acid production by Pseudomonas putida UB1 and its effect as plant growth-promoting rhizobacteria on mustard (Brassica nigra), Agric. Res., 2013, vol. 2, pp. 215−221.
Bhutani, N., Maheshwari, R., Negi, M., and Suneja, P., Optimization of IAA production by endophytic Bacillus spp. from Vigna radiata for their potential use as plant growth promoters, Isr. J. Plant. Sci., 2018, vol. 65, pp. 83−96.
Chesson, P.L. and Warner, R.R., Environmental variability promotes coexistence in lottery competitive-systems, Am. Nat., 1981, vol. 117, pp. 923−943.
Duca, D., Lorv, J., Patten, L.C., Rose, D., and Glick, R.B., Indole-3-acetic acid in plant-microbe interactions, A. van Leeuwenhoek., 2014, vol. 106, pp. 85−125.
Dastager, S.G., Deepa, C.K., Puneet, S.C., Nautiyal, C.S., and Pandey, A., Isolation and characterization of plant growth-promoting strain Pantoea NII-186. from western ghat forest soil, India, Lett. Appl. Microbiol., 2009, vol. 49, pp. 20−25.
Felsenstein, J., Evolutionary trees from DNA sequences: a maximum likelihood approach, J. Mol. Evol. 1981, vol. 17, pp. 68−76.
Felsenstein, J., Confidence limits on phylogenies: an approach using the bootstrap, Evolution, 1985, vol. 39, pp. 783−791.
Flood, J.J. and Copley, D.S., Genome-wide analysis of transcriptional changes and genes that contribute to fitness during degradation of the anthropogenic pollutant pentachlorophenol by Sphingobium chlorophenolicum, mSystems, 2018, vol. 3, pp. 1−16.
Gevers, D., Huys, G., and Swings, J., Applicability of rep-PCR fingerprinting for identification of Lactobacillus species, FEMS Microbiol. Lett.,2001, vol. 205, pp. 31−36.
Giassi, V., Kiritani, C., and Kupper, K.C., Bacteria as growth-promoting agents for citrus rootstocks, Microbiol. Res., 2016, vol. 190, pp. 46−54.
Gomez-Gil, B., Soto-Rodriguez, S., Garcia-Gasca, A., Roque, A., Vazquez-Juarez, R., and Thompson, F.L., Molecular identification of Vibrio harveyi related isolates associated with diseased aquatic organisms, Microbiology, 2004, vol. 150, pp. 1769−1777.
Hansen, H. and Grossmann, K., Auxin-induced ethylene trigger abscisic acid biosynthesis and growth inhibition, Plant Physiol., 2000, vol. 124, pp. 1437−1448.
Hutchinson, G.E., The paradox of the plankton, Am. Nat., 1961, vol. 95, pp. 137−145.
Kalaiselvi, T. and Priya, D., Evaluating the effect of Sphingobium yanoikuyae MH394206 and mixed consortia on growth of rice CO 51 in moisture deficit condition, J. Pharmacogn. Phytochem, 2020, vol. 9, pp. 2016−2021.
Khamwan, S., Boonlue, S., Riddech, N., Jogloy, S., and Mongkolthanaruk, W., Characterization of endophytic bacteria and their response to plant growth promotion in Helianthus tuberosus L., Biocatal. Agric. Biotechnol., 2018, vol. 13, pp. 153−159.
Khanghahi, Y.M., Ricciuti, P., Allegretta, I., Terzano, R., and Crecchio, C., Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions, ESPR, 2018, vol. 25, pp. 25862−25868.
Kirchhorf, G., Reis, V.M., Baldani, J.I., Eckert, B., Döbereiner, J., and Hartmann, A., Occurrence, physiological and molecular analysis of endophytic diazotrophic bacteria in gramineous energy plants, Plant Soil, 1997, vol. 194, pp. 45−55.
Koichiro, T., Glen, S., and Sudhir, K., MEGA11: Molecular Evolutionary Genetics Analysis Version, Mol. Biol. Evol., 2021, vol. 38, pp. 3022−3027.
Konstantinidis, K.T. and Tiedje, J.M, Towards a genome-based taxonomy for prokaryotes, J. Bacteriol., 2005, vol. 187, no. 18, pp. 6258−6264.
Konstantinidis, K.T. and Tiedje, J.M, Genomic insights that advance the species definition for prokaryotes, Proc. Natl. Acad. Sci. U S A., 2005, vol. 102, no. 7, pp. 2567−2572.
Kukavica, B., Motrović, A., Mojović, M., and Jovanović, S.V., Effect of indole-3-acetic acid on pea root growth, peroxidase profiles and hydroxyl radical formation, Arch. Biol. Sci., 2007, vol. 59, pp. 319−326.
Kumari, S., Prabha, C., Singh, A., Kumari, S., and Kiran, S., Optimization of indole-3acetic-acid production by diazotrophic B. subtilis DR2 (KP455653), isolated from rhizosphere of Eagrostis cynosuroides, Int. J. Pharm. Med., 2018, vol. 7, pp. 20−27.
Luziatelli, F., Gatti, L., Ficca, A.G., Medori, G., Silvestri, C., Melini, F., Muleo, R., and Ruzzi, M., Metabolites secreted by a plant-growth-promoting Pantoea agglomerans strain improved rooting of Pyrus communis L. cv. Dar Gazi cuttings, Front. Microbiol., 2020, vol. 11, pp. 1−11.
Maker, O., Kuźniar, A., Patsula, O., Kavulych, Y., Kozlovskyy, V., Wolińska, A., Skórzyńska-Polit, E., Vatamaniuk, O., Terek, O., and Romanyuk, N., Bacterial endophytes of spring wheat grains and the potential to acquire Fe, Cu, and Zn under their low soil bioavailability, Biology, 2021, vol. 10, pp. 1−24.
Meena, V.S., Maurya, B.R., Verma, J.P., and Meena, R.S., Potassium Solubilizing Microorganisms for Sustainable Agriculture, India, Springer, 2016, pp. 10−25.
Normanly, J., Slovin, J.P., and Cohen, J.D., Rethinking auxin biosynthesis and metabolism, Plant Physiol., 1995, vol. 107, pp. 323−329.
Oliveira, C.A., Alves, V.M.C, Marriel, I.E., Gomes, E.A., Scotti, M.R, Careiro, N.P., Guimarăes, C.T., Schaffert, R.E., and Să, N.M.H., Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome, Soil Biol. Biochem., 2009, vol. 41, pp. 1782−1787.
Overbeek, R., Olson, R., Pusch, G.D., Olsen, G.J., Davis J.J., Disz, T., Edwards, R.A., Gerdes, S., Par-rello, B., Shukla, M., Vonstein, V., Wattam, A.R., Xia, F., and Stevens, R., The seed and the rapid annotation of microbial genomes using subsystems technology (RAST), Nucleic Acids Res., 2014, vol. 42, pp. D206−D214.
Patil, N.B., Gajbhiye, M., Ahiwale, S.S., Gunjai, A.B., and Kapadnis, B.P., Optimization of indole 3 acetic acid (IAA) production by Acetobacter diazotrophicus L1 isolated from sugarcane, Int. J. Environ. Sci., 2011, vol. 2, pp. 295−302.
Ramanuj, B.K. and Shelat, N.H., Plant growth promoting potential of bacterial endophytes from medicinal plants. Adv. Res., 2018, vol. 13, pp. 1−15.
Richter, M. and Rossello-Mora, R., Shifting the genomic gold standard for the prokaryotic species definition, Proc. Natl. Acad. Sci. U. S. A., 2009, vol. 106, no. 45, pp. 19126−19131.
Rodrigues, A.A. and Forzani, M.V., Isolation and selection of plant growth-promoting bacteria associated with sugarcane, Pesqui. Agropecu. Trop., 2016, vol. 26, pp. 149−158.
Rodrigues, A.A., Araŭjo, F.V.M., Soares, S.R., Oliveira de F.R.B., Ribeiro, D.A.I., Sibov, T.S., and Vieira, G.J.D., Isolation and prospection of diazotrophic rhizobacteria associated with sugarcane under organic management, An. Acad. Bras. Cienc., 2018, vol. 90, pp. 3813−3829.
Rosenblueth, M. and Martínez-Romero, E., Bacterial endophytes and their interactions with hosts, MPMI, 2006, vol. 19, pp. 827−837.
Saravanan, V.S., Madhaiyan, M., and Thangaraju, M., Solubilization of zinc compounds by the diazothrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus, Chemosphere, 2007, vol. 66, pp. 1794−1798.
Shaik, I., Janakiram, P., Sujatha, L., and Chandra, S., Isolation and identification of IAA producing endosymbiotic bacteria from Gracilaria corticata (J. Agardh), Int. J. Bioassays, 2016, vol. 5, pp. 5179−5184.
Sierra, G., A simple method for the detection of lipolytic activity of micro-organisms and some observation on the influence of the contact between cells and fatty substance, A. van Leeuwenhoek, 1957, vol. 23, p. 15.
Song, C., Wang, W., Gan, Y., Wang, L., Chang, X., Wang, Y., and Yang, W., Growth promotion ability of phosphate-solubilizing bacteria from the soybean rhizosphere under maize soybean intercropping systems, J. Sci. Food Agric., 2022, vol. 102, pp. 1430−1442.
Spaepen, S., Vanderleyden, J., and Remans, R., Indole-3-acetic acid in microbial and microorganism-plant signaling, FEMS Microbiol. Rev., 2007, vol. 31, pp. 425−448.
Stackebrand, E. and Ebers, J., Taxonomic parameters revisited: tarnished gold standards, Microbiol. Today., 2006, vol. 33, pp. 152−155.
Svehla, G., Vogel’s Textbook of Macro and Semimicro Qualitative Inorganic Analysis, London: Longman, 1979.
Tanasupawat, S., Okada, S., and Komagata, K., Lactic acid bacteria found in fermented fish in Thailand, J. Gen. Appl. Microbiol., 1998, vol. 44, pp. 193−200.
Tolieng, V., Booncharoen, A., Nuhwa, R., Thongchul, N., and Tanasupawat, S., Molecular identification, L-lactic acid production, and antibacterial activity of Bacillus strains isolated from soils, J. Appl. Pharm. Sci., 2018, vol. 8, pp. 98−105.
Varghese, N.J., Mukherjee, S., Ivanova, N., Konstantinidis, K.T., Mavrommatis, K., Kyrpides, N.C., and Pati, A., Microbial species delineation using whole genome sequences, Nucleic Acids Res., 2015, vol. 43, pp. 6761−6771.
Versalovic, J., Schneider, M., de Bruijn, F.J., and Lupski, J.R., Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction, Methods Mol. Cell BioI., 1994, vol. 5, pp. 25−40.
Wei, Y.D., Zheng, H., and Hall, J.C., Role of auxinic herbicide-induced ethylene on hypocotyl elongation and root/hypocotyl radial expansion, Pest Manag. Sci., 2000, vol. 56, pp. 377−387.
Welch, M.R. and Graham, D.R., Breeding for micronutrients in staple food crops from a human nutrition perspective, J. Exp. Bot., 2004, vol. 55, pp. 353−364.
Yoon, S.H., Ha, S.M., Kwon, S., Lim, J., Kim, Y., Seo, H., and Chun, J., Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies, Int. J. Syst. Evol. Microbiol., 2017, vol. 67, no. 5, p. 1613.
Yu, J., Yu., H., Fan, G.Q., Wang, G.H., and Liu, X.B., Isolation and characterization of indole acetic acid producing root endophytic bacteria and their potential for promoting crop growth, J. Agric. Sci. Technol., 2016, vol. 18, pp. 1381−1391.
Zhang, T., Hu, F., and Ma, L., Phosphate-solubilizing bacteria from safflower rhizosphere and their effect on seedling growth, Open Life Sci., 2019, vol. 14, pp. 246−254.
ACKNOWLEDGMENTS
This work was supported by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), Graduate School, Chulalongkorn University. We thank the Program in Biotechnology, Faculty of Science, Chulalongkorn University and Faculty of Science and Technology, Suan Sunandha Rajabhat University for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sitlaothaworn, K., Budsabun, T., Booncharoen, A. et al. Diversity of Plant Growth-Promoting Endophytic Bacteria, Genome Analysis of Strain Sx8-8 and Its Rice Germination Promoting Activity. Microbiology 92, 269–283 (2023). https://doi.org/10.1134/S002626172260183X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002626172260183X