Abstract—
The effect of singlet oxygen was studied in the system of Allochromatium (Alc.) vinosum MSU membranes, rose bengal, and light (547−600nm) with and without a quencher. In the system without a quencher, bleaching of the BChl850 band of the LH2 light-harvesting complex was observed and an absorption peak emerged at 698 nm, which belonged to the oxidized product, 3-acetyl-chlorophyll. The efficiency of five quenchers of singlet oxygen for neutralization of the effect of singlet oxygen on bacteriochlorophyll in the light-harvesting complexes of the membranes Alc. vinosum MSU was studied: sodium ascorbate, histidine, imidazole, trolox, and sodium azide. All of them, with the exception of sodium azide, neutralized successively the effect of singlet oxygen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Purple sulfur bacteria may be aerobic or anaerobic, depending on their ability to grow in the presence of oxygen. The first group comprises nonsulfur bacteria, e.g., Rb. sphaeroides, which may grow phototrophically, with accumulation of spheroidene in its cells (complexes), or photoheterotrophically, in which case spheroidene is replaced by spheroidenone with one additional keto group (Takaichi, 2009). The second group comprises sulfur bacteria (Alc. vinosum, T. tepidum, etc.), which are able only of phototrophic growth. These differences evidently stem from the evolution of these bacterial groups in the absence of oxygen or at its increasing concentrations in the atmosphere and in bacterial habitats. While the first group adapted to oxygen conditions and evolved the mechanisms for protection against reactive oxygen species, primarily singlet oxygen, the second group remained restricted to sulfur-containing oxygen-free environments.
BChl, together with carotenoids (additional pigments), is the main pigment of purple photosynthetic bacteria. Both types of the pigments are localized in pigment-protein complexes. Three types of complexes occur usually in the membranes of photosynthetic bacteria: two light-harvesting complexes, LH1 and LH2, and the reaction center (RC). The function of the LH2 and LH1 is absorption of light quanta and transport of this energy as the energy of electron excitation to RC, where primary charge separation occurs (Hoff and Deisenhofer, 1997). Light-harvesting complexes are the main components of the membranes, containing up to 99% of the pigments. They are built from low-molecular mass polypeptides according to a general principle. The main structural module of these complexes is the α/β-heterodimer consisting of two polypeptides, to which BChl molecules (BChl800 and BChl850 in LH2 or BChl870−890 in LH1) and a carotenoid molecule are bound non-covalently (Gabrielsen et al., 2009). The LH2 and LH1 complexes of non-sulfur purple bacteria contain usually 8‒9 and 16 heterodimer pairs, respectively. While the LH2 complex contains 24‒27 BChl molecules and 8‒9 carotenoid molecules, LH1 contains 32 BChl molecules and 16 carotenoid molecules. The LH2 complexes from sulfur bacterium Alc. vinosum have been recently shown to form larger structures than the complexes of non-sulfur bacteria. They consist of 12 heterodimer pairs and contain 36 BChl molecules and 12 carotenoid molecules (Niedzwiedzki et al., 2012; Löhner et al., 2015). BChl850 of these complexes is more easily oxidized by potassium ferricyanide or singlet oxygen than the complexes from non-sulfur bacteria (Makhneva et al., 2016), probably due to the absence of both protective mechanisms and structural modifications resulting in suppressed effect of oxidizers.
After absorption of a light quantum by a BChl molecule and its transition to a long-lived triplet state, it interacts with the oxygen molecule with formation of singlet oxygen. The latter is a strong oxidizing agent, which may oxidize not only BChl, but also other cell components (Imlay, 2003). Effect of high concentrations of both oxygen and singlet oxygen on growth of non-sulfur bacteria has recently become of interest. Fluorescent labels were used to reveal that singlet oxygen not only was produced in the cells of non-sulfur bacteria in the light under oxygen conditions, but also diffuses outside the cell (Kochevar, 2004; Berghoff et al., 2011; Kim et al., 2013). In the cited papers it is considered as a signal molecule able to trigger the synthesis of new proteins, rather than as an oxidizer. Direct assessment of singlet oxygen production by the pigment-protein complexes is rather difficult; thus, it has never been performed for bacterial light-harvesting complexes. Isolated RCs were found to produce singlet oxygen with a low quantum yield (Arellano et al., 2007; Uchoa et al., 2008). Non-sulfur bacteria were studied in above cited papers. Our work with sulfur bacteria indicated that their light-harvesting complexes could be a convenient model for investigation of various aspects of their interaction with singlet oxygen.
The goal of the present work was to assess which quenchers are able to prevent the interaction of singlet oxygen with BChl850 of the LH2 light-harvesting complex from the cells of the sulfur bacterium Alc. vinosum MSU.
MATERIALS AND METHODS
Research subjects and cultivation conditions. The cells of Alc. vinosum MSU (previously Alc. minutissimum) were grown in Larsen’s medium (Kondrat’eva, 1972).
For the isolation of pigment-containing membranes, the cells were resuspended in 0.05 M Tris-HCl buffer (pH 8.0) and treated with a UZDN-0.1-22 sonicator (22 kHz, twice for 60 s). Non-homogenized cells and cell wall residues were removed by differential centrifugation as described by Moskalenko et al. (2005). The obtained membranes were stored at ‒18°C.
Interaction with singlet oxygen. Several types of photosensitizers are routinely used to obtain singlet oxygen. They differ in absorption spectra in the 400‒800-nm region and quantum yield (~20‒75%) (Redmond and Gamlin, 1999; Scholz et al., 2013). In the present work, rose bengal was used, which has certain advantages over other dyes. First, it absorbs in the 440‒580-nm region, masking carotenoids and has no absorption in the absorption region of the product of BChl oxidation (~700 nm), therefore this product may be easily detected by spectroscopy. Second, it has the highest quantum yield (75%) of singlet oxygen compared to other photosensitizers (Redmond and Gamlin, 1999; Scholz et al., 2013). Third, it is well soluble in water. Irradiation of the samples with rose bengal was carried out using a combination of two filters (SZS22 + OS13), which isolated a narrow band at 550‒580 nm, where absorption by BChl and carotenoids was low. Prior to irradiation, the sample was supplemented with rose bengal to the final concentration of 20 µM and then was transferred into a 1-cm spectrophotometric cuvette. All experiments with irradiation were carried out at 24°C in a temperature-controlled cell of a LETI illuminator with a KGM 500 lamp (500 W), at the yellow-green intensity of 2 mW/cm2.
Pigment analysis was carried out by HPLC (Ashikhmin et al., 2014) on an Agilent Zorbax SB-C18 column (Agilent, United States). The HPLC setup consisted of an LC 10ADvp pump with an FCV10 Alvp module, which provided for the solvent gradient at the low-pressure side, an SPD-M20A diode matrix detector, and a CTO-20AC thermostat (Shimadzu, Japan). The chromatograms and absorption spectra of individual pigments were obtained using the LC-solution software (Shimadzu, Japan).
Spectroscopy. Absorption spectra of the membranes were obtained at room temperature using a Cary 50 spectrophotometer (Varian, Australia).
RESULTS AND DISCUSSION
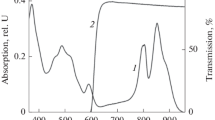
Absorption spectra of the membranes from Alc. vinosum MSU cells contained peaks at 372, 487 (shoulders at 460 and 522), 590, 801, and 852 nm, belonging to the Soret BChl band, carotenoids, Qx, and two Qy transitions of BChl, respectively. Adding of rose bengal masks the maxima of carotenoids and Qx transition (Fig. 1). Illumination of the membranes in the presence of rose bengal results in a 25% decrease of the BChl850 Qy band with its simultaneous shift from 852 to 846 nm. Bleaching of rose bengal was less than 2%. The maximum of 3-acetyl-chlorophyll (700 nm) was almost invisible in absorption spectra and could be observed only on difference spectra (Fig. 1; spectrum 2 and insert, respectively). It should be noted that the light used for irradiation had some effect on these phenomena (>1/3 of total bleaching of the BChl850 band), because the spectral range of the applied filters overlaps with the long-wave slope of carotenoid spectrum. We have previously shown that these pigments were able to initiate BChl photooxidation in the presence of light (Makhneva et al., 2016), acting in fact as photosensitizers a supplementing the action of rose bengal.
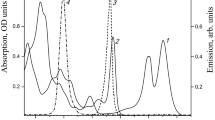
The results of pigment separation for the control membranes of Alc. vinosum MSU are shown on Fig. 2a. BChl and rhodopin were the major pigments in the control sample. It also contained some traces of 3-acetyl-chlorophyll, since no special measures (such as oxygen-free conditions or addition of antioxidants) were taken during cell homogenization and membrane isolation. After illumination of the sample in the presence of rose bengal, a noticeable peak of 3-acetyl-chlorophyll appeared on chromatograms (Fig. 2b), which was in complete agreement with the spectral data. Absorption maxima of BChl and 3-acetyl-chlorophyll in solvents was shifted to the blue range to 770 and 678 nm, respectively (Fig. 2, insert).
Partial HPLC chromatograms of the pigments from the control membranes (a) and from the membranes after 30 min of illumination in the presence of rose 20 µM rose bengal (b). Peak identification: BChl (1), didehydrorhodopin (2), 3-acetyl-chlorophyll (3), rhodopin (4), and spirilloxanthin (5). Insert in (a) shows absorption spectra of BChl (1) and 3-acetyl-chlorophyll (3, increased 5 times). Insert in (b) shows absorption spectra of BChl (1) and 3-acetyl-chlorophyll (3).
It is evident that blocking singlet oxygen should stop BChl oxidation. The results of an experiment carried out in the presence of sodium ascorbate are shown on Fig. 1b. In this experiment, almost no decrease of BChl bands in the near IR range can be observed. The changes in the difference spectrum did not exceed 0.02 opt. U (Fig. 3, spectrum 1), which corresponded to ~2%. Sodium ascorbate is, however, a redox agent, which may reduce, e.g., cytochrome, and its effect is therefore not exactly specific. Other quenchers of singlet oxygen (histidine, imidazole, and trolox) acted similar to sodium ascorbate (Fig. 3, spectra 2‒4). Changes in the BChl spectra in the IR range in the presence of these agents varied from 0.005 to 0.045 opt. U. The difference spectrum of BChl oxidation in the membranes after illumination in the presence of rose bengal without any additives is shown for comparison (Fig. 3, spectrum 6). This spectrum has a minimum at 858 nm and a maximum at 824 nm, which correspond to decrease of the BChl850 band caused by oxidation and to the shift of this band to the short-wave range, respectively. In the presence of quenchers, more long-wave BChl forms bleached (minimum at 865 nm; Fig. 3, spectra 3 and 4). In the course of illumination, small-scale bleaching of the rose bengal absorption band in the range of 550‒570 nm was also observed (Fig. 3).
Difference absorption spectra “spectrum (2) minus spectrum (1)” (see Fig. 1) in the presence of: 100 mM sodium ascorbate (1), 200 mM histidine (2), 200 mM imidazole (3), 200 μM trolox (4), 200 mM NaN3 (5), and without supplements (6). The spectra are shifted along the y axis, the break in spectrum 3 (491‒524 nm) resulted from deletion of significant noise.
All these quenchers showed a clear dependence between decreased BChl850 oxidation and the agent concentration (Fig. 4). The highest effect was observed at the concentrations of trolox, ascorbate, histidine, and imidazole of 0.2, 1‒10, 100‒200, and 200 mM, respectively.
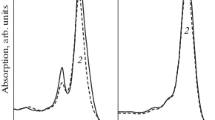
The results obtained with NaN3 (Fig. 3, spectrum 5 and Fig. 5) were completely different from those for other quenchers. NaN3 had almost no effect on BChl850 oxidation at 50 mM (Fig. 5, curve 2), and an increase of NaN3 concentration to 200 mM resulted only in a slight decrease of this effect (by ~3‒5%) after 30 min of illumination. This fact does not agree with the general concept and is presently not understood. NaN3 was repeatedly shown to quench singlet oxygen in the Н2О2‒NaOCl system (Bancirova, 2011), as well as in micelles and liposomes (Miyoshi and Tomita, 1979; Musbat et al., 2013). The quenching efficiency depended directly on the type of structures into which the targets for singlet oxygen were incorporated: while NaN3 did not interact with hydrophobic targets in liposomes, in micelles it was efficient with both hydrophobic and hydrophilic targets (Musbat et al., 2013). The studied membranes resemble liposomes; they contain a lipid bilayer, which is pierced by BChl-containing pigment-protein complexes (hydrophobic targets for singlet oxygen). In our work, NaN3 did not prevent BChl850 oxidation, and our data are therefore in agreement with the results of Musbat et al. (2013). It is, however, still unclear why NaN3 is unable to interact with singlet oxygen immediately after its formation in the hydrophilic phase.
Absorption spectra of the membranes before illumination (1) and after 30-min illumination in the presence of 20 µM rose bengal and 50 mM NaN3 (2) or 200 mM NaN3 (3) (a). Bleaching of the BChl850 in the presence of 20 µM rose bengal (1), +50 mM NaN3 (2), and +200 mM NaN3 (3) depending on illumination time (0–30 min).
Singlet oxygen is a strong destructing factor, which is able to oxidize such various cell components as lipids, proteins, and pigments. Its effect has been usually tested using model systems, which facilitate assessment of formation of the new products. Thus, depending on the solvent type, the interaction of singlet oxygen with diazo dyes resulted in formation of hydroxy- or ketonic groups in the dye molecule with their possible subsequent modification (Bortolus and Monti, 1989). In such plant carotenoids as β-carotene (as well as lutein and zeaxanthin), the action of singlet oxygen in solvents resulted in cleavage of the molecule with formation of fragments of different length and production of the relevant endoperoxide with molecular mass of 192‒416 Da (Rame et al., 2012). Occurrence of a similar process in our samples certainly should have resulted in decreased absorption in the major carotenoid band (Fig. 3) and emergence of new products on HPLC chromatograms, which we have not observed previously (Makhneva et al., 2009). BChl is easily oxidized in model systems with formation of several types of oxidized products, including colorless compounds, bilin-type compounds with an open ring, chlorins, and bacteriochlorins (Smith and Calvin, 1966; Limantara et al., 2006). The main products of oxidation are chlorins, with predominance of 3-acetyl-chlorophyll. The latter was also predominant in our samples and was easily detected by spectrophotometry (Figs. 1 and 3) or by HPLC (Fig. 2). The formation of this product is deals with by the structural features of the BChl molecule, which contains two free protons in positions 7‒8 of the second imidazole ring. If they are not involved in formation of hydrogen bonds with amino acid residues of polypeptides, oxidation releases them from the BChl molecule with a double bond formation, which affects the system of conjugated double bonds. As a result, the long-wave absorption band of the pigment is shifted by ~155 or ~90 nm to the blue region in the complexes and solvents, respectively (Figs. 1 and 2). In our samples, BChl is evidently the main target for singlet oxygen.
In the present work, the effect of five singlet oxygen quenchers (sodium ascorbate, histidine, imidazole, trolox, and NaN3) on neutralization of the effect of singlet oxygen on BChl in light-harvesting complexes of the Alc. vinosum MSU membranes was studied. Except for sodium azide, all of them proved efficient. These quenchers, together with others (β-carotene, α‑tocopherol, ascorbic acid, quercetin, butylhydroxyanisole, butylhydroxytoluene, tetrabutylhydroquinone, betaine, 1,4-diazabicyclo[2.2.2]octane, etc.), are broadly used in experiments with singlet oxygen (Gutiérrez et al., 2001; Dad et al., 2006; Yettella and Min, 2008; Fatima et al., 2016; Wendel et al., 2016). Thus, Fatima et al. (2016) reported that β-carotene, α-tocopherol, ascorbic acid, and quercetin were the most active agents. The authors registered discoloration of nitrosodimethylaniline in the presence of singlet oxygen, which was generated in the system (H2O2 + HOCl → HCl + H2O + 1O2). The results of the cited work (as far as the same quenchers are concerned) are different from our data. In our work, at the same quencher concentrations of trolox (0.1 mM), ascorbate (0.1 mM), imidazole (50 mM), and histidine (50 mM) were ~2.8, ~1.6, ~2, and ~1.9 times more efficient, respectively, compared to the data of Fatima et al. (2016). These differences are rather difficult to explain; they are probably due to the reaction mixture containing hydrogen peroxide (200 mM), which could additionally oxidize nitrosodimethylaniline. We have previously shown that H2O2 affects BChl of Alc. vinosum MSU in a similar manner as chemical oxidizers and singlet oxygen (Moskalenko, 1974).
Singlet oxygen has been long considered a strong damaging factor able to impair the functioning of the cellular compartments (Gorman and Rodgers, 1992). It has been recently, however, considered as a signal molecule as well (Kochevar, 2004; Glaeser and Klug, 2004; Berghoff et al., 2011). In the first case, the cell certainly experiences oxygen stress, i.e., high concentrations of singlet oxygen. It is generated due to external factors or after the degradation of individual pigment-protein complexes and emergence of monomeric BChl, which is a strong sensitizer of singlet oxygen. This effect was originally described by Griffith et al. (1955) and was erroneously ascribed to the protective function of carotenoids. Low concentrations of singlet oxygen may be formed directly inside the cell (Berghoff et al., 2011). Lifetime of singlet oxygen increases sharply on transition from water to phospholipids (membranes). In the case of close contacts between the cells it was hypothesized to migrate (diffuse) from cell to cell (Maisch et al., 2007).
Several methods are now used for determination of singlet oxygen presence or generation in the sample. The first one is direct detection of singlet oxygen using its phosphorescence at 1260 nm. This method was used to reveal production of singlet oxygen by bacterial reaction centers (Arellano et al., 2007; Uchoa et al., 2008). The second is indirect determination of singlet oxygen using traps like histidine or imidazole. It is based on binding of singlet oxygen to the trap and decrease in the total oxygen concentration measured using the Clark electrode (Kraljic and Mohsni, 1978). The third method involves fluorescent traps like Singlet Oxygen Sensor Green (SOSG), which binds singlet oxygen with transition to a fluorescent form emitting at 510‒550 nm (Berghoff et al., 2011). A standard fluorimeter may be used for detection of this form. The possibilities of both photodecomposition and direct generation of singlet oxygen exist, however, for SOSG and other fluorescein-based sensors are exist for both forms (Kim et al., 2013).
Thus, the action of singlet oxygen generated by rose bengal under illumination (547‒600 nm) on the membranes of Alc. vinosum MSU was studied in the absence and presence of singlet oxygen quenchers. In the system without quenchers, bleaching of the BChl850 band of the LH2 light-harvesting complex occurred, and a peak appeared at 698 nm, which belonged to the oxidation product (3-acetyl-chlorophyll. Efficiency of five quenchers (Na ascorbate, histidine, imidazole, trolox, and NaN3) on neutralization of the effect of singlet oxygen on BChl of light-harvesting complexes of the membranes of Alc. vinosum MSU was studied. All of them, except for sodium azide, neutralized this effect successfully.
The approach used in the present work is a simple method for detection of singlet oxygen by changes in absorption spectra of Alc. vinosum MSU membranes. It will be used for research on various aspects of the interaction between singlet oxygen and the pigments of photosynthetic bacteria.
REFERENCES
Arellano, J.B., Yousef, Y.A., Melø, T.B., Mahamad, S.B., Cogdell, R.J., and Naqvi, K.R., Formation and geminate quenching of singlet oxygen in purple bacterial reaction center, J. Photochem. Photobiol., 2007, vol. 87, pp. 105‒112.
Ashikhmin, A., Makhneva, Z., and Moskalenko, A., The LH2 complexes are assembled in the cells of purple sulfur bacterium Ectothiorhodospira haloalkaliphila with inhibition of carotenoid biosynthesis, Photosynth. Res., 2014, vol. 119, pp. 291–303.
Bancirova, M., Sodium azide as a specific quencher of singlet oxygen during chemiluminescent detection by luminol and Cypridina luciferin analogues, Luminescence, 2011, vol. 26, pp. 685‒688.
Berghoff, B.A., Glaeser, J., Nuss, A.M., Zobawa, M., Lottspeich, F., and Klug, G., Anoxygenic photosynthesis and photooxidative stress: a particular challenge for Roseobacter, Environ. Microbiol., 2011, vol. 13, pp. 775‒791.
Bortolus, P. and Monti, S., Physical quenching and chemical reaction of singlet molecular oxygen with Azo dyes, J. Org. Chem, 1989, vol. 54, pp. 534‒540.
Dad, S., Bisby, R.H., Clark, I.P., and Parker, A.W., Formation of singlet oxygen from solutions of vitamin E, Free Radic. Res., 2006, vol. 40, pp. 333‒338.
Fatima, K., Masood, N., and Luqman, S., Quenching of singlet oxygen by natural and synthetic antioxidants and assessment of electronic UV/Visible absorption spectra for alleviating or enhancing the efficacy of photodynamic therapy, Biomed. Res. Therap., 2016, vol. 3, pp. 514‒527.
Gabrielsen, M., Gardiner, A.T., and Cogdell, R.J., Peripheral complexes of purple bacteria, in The Purple Phototrophic Bacteria, Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty J.T., Eds., Adv. Photosynth. Respir., Dordrecht: Springer, 2009, vol. 28, pp. 135‒153.
Glaeser, J. and Klug, G., Photo-oxidative stress in Rhodobacter sphaeroides: protective role of carotenoids and expression of selected genes, Microbiology (UK), 2005, vol. 151, pp. 1927‒1938.
Gorman, A.A. and Rodgers, M.A.J., Current perspectives of singlet oxygen detection in biological environments, J. Photochem. Photobiol. B: Biol., 1992, vol. 14, pp. 159‒176.
Griffith, M., Sistrom, W.R., Cohen-Bazire, G., and Stanier, R.Y., Functions of carotenoids in photosynthesis, Nature, 1955, vol. 176, pp. 1211‒1214.
Gutiérrez, I., Criado, S., Bertolotti, S., Norman, A., and Garcia, N., Dark and photoinduced interactions between trolox, a polar-solvent-soluble model for vitamin E, and riboflavin, J. Photochem. Photobiol., 2001, vol. 62, pp. 133‒139.
Hoff, A.J. and Deisenhofer, J., Photophysics of photosynthesis. Structure and spectroscopy of reaction centers of purple bacteria, Physics Rep., 1997, vol. 287, pp. l‒247.
Imlay, J.A., Pathways of oxidative damage, Annu. Rev. Microbiol., 2003, vol. 57, pp. 395‒418.
Kim, S., Fujitsuka, M., and Majima, T., Photochemistry of singlet oxygen sensor green, J. Phys. Chem. B, 2013, vol. 117, pp. 13985‒13992.
Kochevar, I.E., Singlet oxygen signaling: from intimate to global, Sci. STKE, 2004, vol. 2004, pp. 1‒3.
Kondratyeva Ye.N., Fotosinteziruyushchie bakterii i bakterial’nyi fotosintez (Photosynthetic Bacteria and Bacterial Photosynthesis), Moscow: USSR Acad. Sci., 1972.
Kraljic, I. and Mohsni, S.E.L., A new method for the detection of singlet oxygen in aqueous solutions, Photochem. Photobiol., 1978, vol. 28, pp. 577‒581.
Krieger-Liszkay, A. and Trebst, A., Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre, J. Exp. Botany, 2006, vol. 57, pp. 1677‒1684.
Limantara, L., Koehler, P., Wilhelm, B., Robert, J., Porra, R.J., and Scheer, H., Photostability of bacteriochlorophyll a and derivatives: potential sensitizers for photodynamic tumor therapy, Photochem. Photobiol., 2006, vol. 82, pp. 770‒780.
Löhner, A., Carey, A.M., Hacking, K., Picken, N., Kelly, S., Cogdell, R., and Köhler, J., The origin of the split B800 absorption peak in the LH2 complexes from Allochromatium vinosum, Photosynth. Res., 2015, vol. 123, pp. 23‒31.
Maisch, T., Baier, J., Franz, B., Maier, M., Landthaler, M., Szeimies, R.M., and Bäumler, W., The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria, Proc. Natl. Acad. Sci. U. S. A., 2007, vol. 104, pp. 7223–7228.
Makhneva, Z.K., Ashikhmin, A.A., Bolshakov, M.A., and Moskalenko, A.A., 3-Acetyl-chlorophyll formation in light harvesting complexes of purple bacteria by chemical oxidation, Biochemistry (Moscow), 2016, vol. 81, pp. 176‒186.
Makhneva, Z.K., Bolshakov, M.A., Ashikhmin, A.A., Erokhin, Y.E., and Moskalenko, A.A. Influence of blue light on the structure stability of antenna complexes from Allochromatium minutissimum with different content of carotenoids, Biochemistry Suppl. Ser. A: Membrane and Cell Biol., 2009, vol. 3, no. 2, pp. 123–127.
Miyoshi, N. and Tomita, G., Quenching of singlet oxygen by sodium azide in reversed micellar systems, Z. Naturforsch., 1979, vol. 34b, pp. 339‒343.
Moskalenko, A.A., Makhneva, Z.K., Fiedor, L., and Scheer, H., Effects of carotenoid inhibition on the photosynthetic RC–LH1 complex in purple sulphur bacterium Thiorhodospira sibirica, Photosynth. Res., 2005, vol. 86, pp. 71–80.
Moskalenko, A.A., Pigment-protein complexes and their interaction in the structures of bacterial and plant photosynthetic apparatus, Doctoral (Biol.) Dissertation, Pushchino: IPFS RAN, 1993.
Musbat, L., Weitman, H., and Ehrenberg, B., Azide quenching of singlet oxygen in suspensions of microenvironments of neutral and surface charged liposomes and micelles, Photochem. Photobiol., 2013, vol. 89, pp. 253‒258.
Niedzwiedzki, D.M., Bina, D., Picken, N., Honkanen, S., Blankenship, R.E., Holten, D., and Cogdell, R.J., Spectroscopic studies of two spectral variants of light-harvesting complex 2 (LH2) from the photosynthetic purple sulfur bacterium Allochromatium vinosum, Biochim. Biophys. Acta, 2012, vol. 1817, pp. 1576–1587.
Ohara, K., Kikuchi, K., Origuchi, T., and Nagaoka, S., Singlet oxygen quenching by trolox C in aqueous micelle solutions, J. Photochem. Photobiol. B, 2009, vol. 97, pp. 132‒137.
Rame, F., Birtic, S., Cuiné, S., Christian Trianta-phylidés, C., Ravanat, J.L., and Havaux, M., Chemical quenching of singlet oxygen by carotenoids in plants, Plant Physiol., 2012, vol. 158, pp. 1267‒1278.
Redmond, R.W. and Gamlin, J.N., A compilation of singlet oxygen yields from biologically relevant molecules, Photochem. Photobiol., 1999, vol. 70, pp. 391‒475.
Scholz, M., Roman Dedic, R., Breitenbach, T., and Hala, J., Singlet oxygen-sensitized delayed fluorescence of common watersoluble photosensitizers, Photochem. Photobiol. Sci., 2014, vol. 13, pp. 1203‒1212.
Smith, J.R. and Calvin, M., Studies on the chemical and photochemical oxidation of bacteriochlorophyll, J. Am. Chem. Soc., 1966, vol. 88, pp. 4500‒4506.
Takaichi, S., Distribution and biosynthesis of carotenoids, in The Purple Phototrophic Bacteria, Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty J.T., Eds., Adv. Photosynth. Respir., Dordrecht: Springer, 2009, vol. 28, pp. 97‒117.
Uchoa, A.F., Knox, P.P., Turchielle, R., Seifullina, N.Kh., and Baptista, S.M., Singlet oxygen generation in the reaction centers of Rhodobacter sphaeroides, Eur. Biophys., 2008, vol. 37, pp. 843‒850.
Wendel, M., Nizinski, S., Gierszewski, M., Prukala, D., Sikorski, M., Starzak, K., Wybraniec, S., and Burdzin-ski, G., Chemical quenching of singlet oxygen by betanin, Photochem. Photobiol. Sci., 2016, vol. 15, pp. 872‒878.
Yettella, R.R. and Min, D.B., Quenching mechanisms and kinetics of trolox and ascorbic acid on the riboflavin-photosensitized oxidation of tryptophan and tyrosine, J. Agric. Food Chem., 2008, vol. 56, pp. 10887‒10892.
ACKNOWLEDGMENTS
The work was partially supported by the Russian Foundation for Basic Research, projects nos. 18-04-00684_a, 18-34-00416_mol_a, and 17-04-00929_a). The results presented on Figs. 1‒3 were obtained within the framework of the State Task no. AAAA-A17-117030110140-5.
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by P. Sigalevich
.
Rights and permissions
About this article
Cite this article
Makhneva, Z.K., Ashikhmin, A.A., Bolshakov, M.A. et al. Quenchers Protect BChl850 from Action of Singlet Oxygen in the Membranes of a Sulfur Photosynthetic Bacterium Allochromatium vinosum Strain MSU. Microbiology 88, 79–86 (2019). https://doi.org/10.1134/S0026261719010119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261719010119