Abstract
The kinetics of mineralization of oxalic acid Н2С2О4 under the action of ozone in acidic aqueous solution (С(НClО4) = 0.1 M, pH ~ 1) with the addition of \({\text{MnO}}_{4}^{ - }\) or Mn2+ ions was studied. It was found that manganese ions are effective catalysts for the reaction of O3 with oxalic acid. Regardless of the manganese species (\({\text{MnO}}_{4}^{ - }\) or Mn2+) added to the solution, it was converted into an oxalate complex of tetravalent manganese in the course of the reaction, and this complex was the stable form of the catalyst in the system under consideration. The kinetics of release of carbon dioxide—a product of the reaction of Н2С2О4 with О3—was determined depending on the concentrations of ozone in the gas stream and oxalic acid and manganese in the solution. A basic scheme of the catalysis of the test reaction by manganese ions was proposed, and a kinetic model was constructed to adequately describe the experimental results. The reaction scheme is based on the fact that oxalate is oxidized to CO2 in the course of a complex decomposition reaction of the oxalate complex of tetravalent manganese; in this case, Mn(IV) is reduced to Mn2+. The complex is regenerated by the oxidation of Mn2+ to Mn(IV) with ozone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The removal of oxalic acid and its salts, oxalates, from various solutions is required for the purification of process water and wastewater of various industries [1–3]. The oxidative mineralization of oxalic acid is widely used in industry for the processing of spent nuclear fuel [4–6]. At the same time, ozone is an optimal oxidizing agent because it can be obtained on site, and no additional waste is generated upon its use [7]. However, oxalic acid and oxalates do not directly interact with the O3 molecule [8]. Oxalate oxidation in conventional ozonation can occur due to side reactions, mainly under the action of the free hydroxyl radical OH•, an intermediate substance in a complex self-decomposition reaction of ozone in an aqueous solution [3]. These processes most noticeably occur in an alkaline medium at high temperatures [7, 9], and their contribution is usually insignificant in acidic solutions. Thus, a study of the catalytic ozonation of oxalic acid is of considerable current interest.
It is well known that manganese ions are effective catalysts for ozonation processes. As a rule, their addition leads to an acceleration of the mineralization of organic impurities [10–14]; however, in some cases, the catalytic effect is not observed [15, 16].
The catalysis of oxalic acid ozonation by manganese ion has not been adequately studied. Intermediates and active forms of the catalysts have not been identified, but there are various assumptions on their nature (for example, [10]). Catalysis by permanganate ions has not been studied, although their catalytic action can be expected. Indeed, permanganate ions effectively react with Н2С2О4 (see [5]); on the other hand, ozone can oxidize manganese ions in lower oxidation states to permanganate [17, 18] and thus regenerate the catalyst. In addition, the ozonation of oxalic acid solutions of relatively high concentrations in the presence of strong acids, which is currently important for the processing of spent nuclear fuel, has almost not been studied.
The aim of this work was to experimentally study the kinetics of homogeneous catalytic oxidation of oxalic acid to carbon dioxide on the ozonation of its acidic aqueous solutions, to identify intermediate compounds, and to determine the mechanism (kinetic scheme) of the catalytic reaction. Permanganate ions (\({\text{MnO}}_{4}^{ - }\)) or divalent manganese ions (Mn2+) were used as the initial forms of the catalysts.
EXPERIMENTAL
The experiments were performed using a setup described previously [19]. The interaction between ozone and oxalic acid solutions was carried out in a bubble column reactor at room temperature (20 ± 1°С). The initial solutions contained 0.02–0.2 M oxalic acid, 0.1 M perchloric acid, and 3 × 10–5–4.2 × 10–4 M potassium permanganate or manganese sulfate. Distilled water, concentrated chemically pure perchloric acid, chemically pure oxalic acid dihydrate, manganese(II) sulfate pentahydrate of analytical grade, and pharmacopoeial potassium permanganate were used to prepare these solutions. The reactor had a tap for sampling the reaction solution. The UV–visible spectra of liquid samples in a range of 190–1100 nm were recorded on an Agilent-8453 spectrophotometer (Agilent Technologies, the United States).
Ozone was synthesized in a barrier discharge ozonator from pure oxygen gas. The ozone concentration in the gas flow was measured at the inlet and outlet of the reactor using Medozon-254/5 photometric ozone meters (Medozon, Russia); the inlet concentration ranged from 10 to 40 g/m3 in various experiments. The rate of ozone absorption (consumption) in the reactor (mol L–1 min–1) was found from the ratio
where v is the volumetric flow rate of the ozone–oxygen mixture; Vreaction is the volume of the reaction solution; C(O3), mol/L, is the ozone concentration at the reactor outlet; and C°(O3), mol/L, is the ozone concentration at the reactor outlet measured in similar experiments when the reactor contained only a 0.1 M solution of HClO4. The use of C°(O3) instead of the inlet ozone concentration Cin(O3) was substantiated previously [20]. In special experiments, it was determined that C°(O3) = 0.95 × Cin(O3). In all of the experiments, the gas flow rate and the reaction solution volume were v = 0.35 L/min and Vreaction = 0.2 L, respectively.
The carbon dioxide (СО2) formed upon the oxidation of oxalic acid was quantitatively determined based on the neutralization time (∆t, min) of a solution of NaOH [19]. The gases leaving the reactor were passed through a furnace heated to a temperature of ~500°С in order to decompose ozone; this procedure ensured almost complete removal of О3 [21]. Then, they entered a trap filled with 100 mL of a 0.01–0.001 M NaOH solution (prepared from chemically pure sodium hydroxide) with the addition of a phenolphthalein indicator according to Levanov et al. [19]. To accurately determine the neutralization time, the solution samples were taken from the trap at regular intervals, and their optical density at the absorption maximum of the colored form of phenolphthalein at 552 nm was measured on a KFK-3 photometer (OAO Zagorsk Optical-Mechanical Plant, Russia); after the measurements, the samples were returned. The rate of СО2 release (mol L–1 min–1) was calculated from the formula
where VNaOH = 0.1 L is the volume of sodium hydroxide solution in the trap, and CNaOH is its concentration, M. The relative errors in the determination of r(O3) and r(CO2) were 10–15%.
The rates of ozone absorption and carbon dioxide evolution were found in the stationary operation mode of the reactor. This mode was established due to the fact that the reactor is a flow-through one, and the experimental parameters, in particular, gas flow and ozone supply rates, remained unchanged with time. Strictly speaking, the mode was approximately stationary because oxalic acid was irreplaceably consumed in the course of the reaction. However, it was present in a large excess, and a decrease in its concentration in the course of the experiment (no more than 3% of the initial value) can be neglected.
RESULTS AND DISCUSSION
On the ozonation of oxalic acid solutions in the absence of manganese salts, the rate of carbon dioxide evolution was lower than the limit of detection (<1 × 10–5 mol L–1 min–1). The addition of potassium permanganate or manganese sulfate to the reaction solution led to the oxidation of oxalic acid under the action of ozone and to a significant release of CO2. Figures 1–4 (points) illustrate the experimental data. The rate of СО2 evolution was directly proportional to the concentration of ozone in the initial gases (Fig. 1); the dependences of r(CO2) on the concentrations of oxalic acid (Fig. 2) and manganese ions (Fig. 3) were more complicated, and they are discussed below. Note that the catalytic effect of manganese salts was independent of the initial chemical species, \({\text{MnO}}_{4}^{ - }\) or Mn2+, added to the solution.
Figure 4 shows the ratio between the rates of СО2 evolution and О3 consumption; for all of the experiments performed in this work, it varied within a range of 0.6–1.1. The value of r(CO2)/r(O3) tended to increase with the catalyst concentration. The stoichiometric equation for the oxidation reaction of oxalic acid with ozone is
If ozone was consumed only in the target reaction, the ratio r(CO2)/r(O3) should be 2. Smaller values of r(CO2)/r(O3) found in our experiments indicate the occurrence of side ozone reactions that did not lead to the oxidation of H2C2O4.
An analysis of the spectra of reaction solutions recorded at different times after the onset of ozonation (Figs. 5, 6) showed that, regardless of which initial manganese ions (\({\text{MnO}}_{4}^{ - }\) or Mn2+) were taken, they were converted into the same new compound. Its spectrum was characterized by a high-intensity shoulder at 385–390 nm (Figs. 5, 6) and a less intense peak with a maximum at 645 nm and a shoulder at 735 nm (Fig. 7). A comparison with published spectra [22] of the oxalate complex of tetravalent manganese (Fig. 7) demonstrated that manganese ions in our solutions were converted precisely into this complex. Its structure has not been finally established, and the chemical formulas [Mn(C2O4)2(OH)2]2– [23] and [(C2O4)2MnO2Mn(C2O4)2]4– [22] were attributed to it; in this work, we will write the formula as MnO(C2O4)\(_{2}^{{2 - }}\). The stability constant of the complex was not reported in the literature; however, this complex was easily formed in the presence of Mn(IV) and oxalate ions in the solution [22–24] to be indicative of a high value of this constant. Yukichi et al. [22] estimated the molar absorption coefficients of the complex in order to determine its concentration in solution. It was found that, under the conditions of our experiments in a stationary mode, from 85 to 100% of manganese occurred as MnO(C2O4)\(_{2}^{{2 - }}\); that is, this complex ion was the predominant chemical species of manganese in our reaction solutions.
Superposition of the spectra of a reaction solution of 0.05 M H2C2O4 + 0.1 M HClO4 + 4 × 10–4 M Mn2+ in the course of ozonation (Cin(O3) = 27–28 g/m3) and the complex MnO(C2O4)\(_{2}^{{2 - }}\) according to Yoshino et al., 1974 [22].
It is well known that divalent and trivalent manganese ions can also form stable oxalate complexes. We calculated the equilibrium composition of Mn(II) and Mn(III) solutions, which are similar in acidity and oxalate content to our reaction solutions, using reference data on the stability constants of complexes [25] and the dissociation constant of oxalic acid [26]. The calculation results showed that the main form of divalent manganese was the free ion (aqua complex) Mn2+(aq) (its fraction was more than 94% of total Mn(II)), and the main form of trivalent manganese was the complex ion MnO(C2O4)\(_{2}^{ - }\) (more than 96% of total Mn(III)). The spectrum of MnO(C2O4)\(_{2}^{ - }\) is well known [27], and it allows one to identify this ion in aqueous solutions. The MnO(C2O4)\(_{2}^{ - }\) ions were not detected in our reaction solutions.
The oxalate complex of tetravalent manganese MnO(C2O4)\(_{2}^{{2 - }}\) in aqueous solutions is unstable, and it undergoes decomposition with the oxidation of the ligand—oxalate ion—to СО2 and the reduction of Mn(IV) to Mn(III) and further to Mn(II) [22–24]:
We have performed preliminary experiments to study the kinetics of decomposition of MnO(C2O4)\(_{2}^{{2 - }}\) in our reaction solutions. In the presence of ozone, the complex disappeared according to a reaction of the zero kinetic order, and the spectral signals of trivalent manganese (the oxalate complex MnO(C2O4)\(_{2}^{ - }\)) were not detected; the end product was Mn2+(aq). Determination of the mechanism of decomposition requires special studies, which are planned to be carried out in the future.
The data obtained allowed us to propose the following basic scheme for the catalysis of a reaction of oxalic acid with ozone by manganese ions under the conditions of our experiments (in the steady-state mode). Oxalate is oxidized to carbon dioxide in the course of a complex decomposition reaction of the tetravalent manganese complex MnO(C2O4)\(_{2}^{{2 - }}\); in this case, Mn(IV) is reduced to Mn2+ rather than Mn(II). The distribution of electron density in the oxalate ion bound as a ligand is significantly changed, in comparison with that in the free ions C2O\(_{4}^{{2 - }}\) and HC2O\(_{4}^{ - }\) and the molecule H2C2O4, to facilitate its interaction with oxidants. Therefore, we can assume the possibility of the rapid oxidation of an oxalate ligand in the complex MnO(C2O4)\(_{2}^{{2 - }}\) under the action of ozone.
Divalent manganese ions are quickly oxidized with ozone to a tetravalent state according to the reaction
The interaction of Mn2+ with O3 has been studied in sufficient detail [17, 28, 29]. It was concluded [17, 29] that it occurs by the mechanism of oxygen atom transfer, and the primary product is the MnO2+ ion. The rate constant of reaction (I) in aqueous solutions acidified with perchloric acid at 20°С is kI = 1500 L mol–1 s–1 [29].
With an excess of oxalic acid, tetravalent manganese ions immediately form an oxalate complex:
after which the catalytic cycle is repeated.
Andreozzi et al. [10] also studied the catalytic ozonation of acidified oxalic acid solutions with the addition of Mn2+ ions, but they did not identify manganese compounds in the reaction system. On the basis of indirect data, Andreozzi with coauthors [10, 11] assumed that oxalates were oxidized upon interacting with trivalent manganese (possibly, within the oxalate complex of Mn(III)). In this work, we found that the main active catalyst species is the oxalate complex of Mn(IV), while Mn(III) compounds were not detected.
Based on the above reaction scheme, we simulated the kinetics of СО2 evolution and О3 consumption in the ozonation of oxalic acid solutions in the stationary mode of our experiments. Note that the model is simplified, and it takes into account only the main processes. Due to the lack of information, the detailed mechanism of decomposition of the oxalate complex of tetravalent manganese MnO(C2O4)\(_{2}^{{2 - }}\) is not discussed. The kinetics of decomposition of MnO(C2O4)\(_{2}^{{2 - }}\) was described by formal kinetic relations, and two possible versions of the course of this process were considered.
Version A with the participation of ozone | Version B without the participation of ozone | |
Stoichiometric equation of the decomposition reaction | ||
MnO(C2O4)\(_{2}^{{2 - }}\) + О3 + 4H+ → Mn2+ + 4CO2 + 2H2O + О2 | MnO(C2O4)\(_{2}^{{2 - }}\) + 2H+ → Mn2+ + 2CO2 + C2O\(_{4}^{{2 - }}\) + H2O | (III) |
Expressions for the rates of decomposition of the complex and release of CO2 | ||
rIII = kIII[MnO(C2O4)\(_{2}^{{2 - }}\)][О3][H+]n r(СО2) = 4rIII | rIII = kIII[MnO(C2O4)\(_{2}^{{2 - }}\)][H+]n r(СО2) = 2rIII | (1) |
Here, kIII is the effective rate constant of complex reaction (III); n is the kinetic order with respect to the concentration of H+ ions; and [X] denotes the concentration of substance X in solution. The possible values of n = 0, 1, 2,… were considered in the simulation.
The complex ion MnO(C2O4)\(_{2}^{{2 - }}\) is the leading intermediate in our model because oxalate is oxidized and CO2 is formed in the course of its decomposition. Its concentration was calculated in a quasi-equilibrium approximation from the condition of equilibrium in reaction (II) of the formation of this complex
the conditions of steady-state concentration of tetravalent manganese in the reaction solution (equality of the rates of formation and consumption of Mn (IV))
and the material balance equations for manganese
Here, KII is the equilibrium constant of reaction (II), and C(Mn) is the total concentration of manganese ions in the reaction solution, which is equal to the initial concentration of potassium permanganate or manganese sulfate. The solution of a system of Eqs. (2)–(4) led to the following expressions for the concentration:
The rate of carbon dioxide evolution was calculated using formulas (1), into which relations (5) were substituted.
The concentration of dissolved ozone [O3] was determined from the system of equations
which reflect the material balance and equality of the rates of dissolution and consumption of ozone in the stationary mode of the reactor. In Eq. (6), kLa is the volumetric coefficient of mass transfer of ozone; \({{H}_{{{{{\text{O}}}_{{\text{3}}}}}}}\) is the true Henry’s constant of ozone (dimensionless), which is equal to the ratio between ozone concentrations in the solution and in the gas phase expressed in mol/L at equilibrium that would be observed in the absence of chemical reactions of ozone; and \({{k}_{{{{{\text{O}}}_{{\text{3}}}}}}}\) is the specific rate of chemical reactions of ozone in solution. The value of kLa = 0.1 s–1 was determined by Levanov et al. [20] for similar experimental conditions and the same reactor as that used in this work. The value of \({{H}_{{{{{\text{O}}}_{{\text{3}}}}}}}\) = 0.23 under the conditions of our experiments was estimated based on published data [30]. The expression for calculating the concentration of ozone in solution is
In the model, the rate of ozone reactions in solution is described by the relations
where the term kd[O3] takes into account all side reactions that do not lead to oxalate oxidation, and kd denotes the effective rate constant of these reactions. The reaction rate can be expressed in terms of the concentration of the leading intermediate MnO(C2O4)\(_{2}^{{2 - }}\) using steady-state conditions (3) as follows:
The corresponding expressions for the specific rate of ozone reactions are the following:
It should be noted that the system of Eqs. (6) is valid in the case when the consumption of ozone at the gas–liquid interface can be neglected in comparison with the reactions of ozone in the main volume of the reaction solution. Indeed, we can demonstrate that this actually took place in the experiments of this work, similarly to how it was done previously [31].
The concentrations of Н+ ions, undissociated Н2С2О4 oxalic acid molecules, and HC2O\(_{4}^{ - }\) hydroxalate and C2O\(_{4}^{{2 - }}\) oxalate anions in the reaction solution were calculated on the basis of a quasi-equilibrium approximation by solving a system of algebraic equations, which included the expressions for the equilibrium constants of acid dissociation in an aqueous solution
the material balance equations for oxalate
and the charge conservation conditions
Here, Ka1 and Ka2 are the first and second dissociation constants of oxalic acid (their values under the conditions of our experiments were estimated from published data [26]), and С(Н2С2О4) and С(НClО4) are the acidimetric concentrations of oxalic and perchloric acids, respectively, in the reaction solution.
Expressions (5), (7), (8), and (10)–(12) form a system of algebraic equations, which allowed us to calculate the concentrations of participants in the complex catalytic reaction of oxalic acid with ozone in our solution in a stationary mode. The sequence of actions in the calculation was the following: First, the concentrations of Н+, Н2С2О4, HC2O\(_{4}^{ - }\), and C2O\(_{4}^{{2 - }}\) were determined by solving Eqs. (10)–(12); in this case, the consumption of oxalic acid in the course of ozonation was neglected. Then, the concentrations of O3 and MnO(C2O4)\(_{2}^{{2 - }}\) in the solution were found from Eqs. (7), (8), and (5), and the equations were solved by a simple iteration method using Microsoft Excel. Next, the rates of carbon dioxide evolution and the rate of ozone consumption were calculated using formulas (1) and expressions (8), respectively.
The undetermined parameters of the model were the values of KII, kIII, n, and kd. The values of KII, kIII, and n were determined from the condition of the best coincidence of the experimental and calculated rates of СО2 evolution. The value of kd was estimated based on the agreement between the experimental and calculated ratios between the rates of CO2 evolution and O3 consumption.
The directly proportional dependence of r(CO2) on the concentration of ozone in the incoming gas flow can be reproduced only for version A of the decomposition of the leading intermediate MnO(C2O4)\(_{2}^{{2 - }}\) with the participation of ozone. In the calculations according to version B, the dependence of r(CO2) on Cin(O3) is obtained in the form of a nonlinear increasing curve rapidly reaching saturation, which contradicts the experimental results (see Fig. 1). Therefore, only version A is considered below.
The calculated rates of СО2 evolution reached experimental values only for n = 0 or 1. At the optimal values of the remaining constants kd, KII, and kIII, the calculated dependence of r(CO2) on С(Н2С2О4) was closer to the experimental data at n = 1; thus, we obtained the optimal value n = 1. The optimal value kd = 3 × 10–2 s–1 was found on the basis that the calculated ratio r(CO2)/r(O3) corresponded to the experimental range 0.6–1.1 under similar experimental conditions.
Figures 1–3 compare the experimental and calculated rates of carbon dioxide evolution for various experimental conditions at the optimal values of the model parameters n = 1, kd = 3 × 10–2 s–1, KII = 1 × 1011 M–2, and kIII = 390 L2 mol–2 s–1. It can be seen that the calculated and experimental results are in good agreement (with consideration for errors in the experimental data). In this case, the model correctly reproduced the qualitative form of the experimental curves, including the nonlinear dependences of r(CO2) on the concentrations of oxalic acid (Fig. 2) and manganese ions (Fig. 3). This fact confirms that the reaction scheme and the kinetic model correctly describe the main chemical processes in the catalysis of oxalic acid oxidation by ozone with manganese salts under the conditions of our experiments. The high value of the parameter KII, the equilibrium constant of reaction (II), is consistent with published experimental data [22–24], according to which oxalate and Mn(IV) very easily form a complex compound with each other.
CONCLUSIONS
Thus, in this work, we found effective catalysis of the reaction of oxalic acid with ozone by manganese ions in acidic solutions and studied for the first time the catalytic action of permanganate ions. We demonstrated that, regardless of the starting manganese compounds (MnO4– or Mn2+) taken, they were converted into the same active form of the catalyst—the oxalate complex of tetravalent manganese. This complex was identified for the first time in the ozone–oxalic acid–manganese ions system. We proposed a reaction scheme and the corresponding kinetic model of the catalytic reaction to adequately describe the experimental kinetics and explain the catalytic action of manganese ions.
REFERENCES
Marcì, G., García–López, E., and Palmisano, L., J. Appl. Electrochem., 2008, vol. 38, no. 7, p. 1029.
Bangun, J. and Adesina, A.A., Appl. Catal., A, 1998, vol. 175, no. 1, p. 221.
Von Sonntag, C. and Von Gunten, U., Chemistry of Ozone in Water and Wastewater Treatment. From Basic Principles to Applications, London: IWA, 2012.
Michael, K.M., Rizvi, G.H., Mathur, J.N., and Ramanujam, A., J. Radioanal. Nucl. Chem., 2000, vol. 246, no. 2, p. 355.
Ganesh, S., Desigan, N., Chinnusamy, A., and Pandey, N.K., J. Radioanal. Nucl. Chem., 2021, vol. 328, no. 3, p. 857.
Anan’ev, A.V., Tananaev, I.G., and Shilov, V.P., Russ. Chem. Rev., 2005, vol. 74, no. 11, p. 1039.
Seliverstov, A.F., Lagunova, Yu.O., Ershov, B.G., and Shashkovskii, S.G., Russ. J. Gen. Chem., 2017, vol. 87, no. 11, p. 2533.
Hoigné, J. and Bader, H., Water Res., 1983, vol. 17, no. 2, p. 185.
Gogolev, A.V., Shilov, V.P., Garnov, A.Yu, and Anan’ev, A.V., Radiochemistry, 2006, vol. 48, no. 1, p. 31.
Andreozzi, R., Insola, A., Caprio, V., and D’amore, M.G., Water Res., 1992, vol. 26, no. 7, p. 917.
Andreozzi, R., Caprio, V., D’amore, M.G., and Insola, A., Environ. Technol., 1995, vol. 16, no. 9, p. 885.
Andreozzi, R., Caprio, V., Insola, A., Marotta, R., and Tufano, V., Water Res., 1998, vol. 32, no. 5, p. 1492.
Ma, J. and Graham, N.J.D., Water Res., 2000, vol. 34, no. 15, p. 3822.
Qin, W., Tan, P., Song, Y., Wang, Z., Nie, J., and Ma, J., Sep. Purif. Technol., 2021, vol. 261, p. 118272.
Xiao, H., Liu, R., Zhao, X., and Qu, J., J. Mol. Catal. A: Chem., 2008, vol. 286, no. 1, p. 149.
Dong, Y., Yang, H., He, K., Song, S., and Zhang, A., Appl. Catal., B, 2009, vol. 85, no. 3, p. 155.
Reisz, E., Leitzke, A., Jarocki, A., Irmscher, R., and Von Sonntag, C., J. Water Supply: Res. Technol.–AQUA, 2008, vol. 57, no. 6, p. 451.
Levanov, A.V., Kuskov, I.V., Antipenko, E.E., and Lunin, V.V., Russ. J. Phys. Chem., 2006, vol. 80, no. 4, p. 556.
Levanov, A.V., Isaikina, O.Y., and Kharlanov, A.N., Russ. J. Phys. Chem. A, 2020, vol. 94, no. 11, p. 2219.
Levanov, A.V., Isaikina, O.Y., Gasanova, R.B., and Lunin, V.V., Russ. J. Phys. Chem. A, 2017, vol. 91, no. 8, p. 1427.
Levanov, A.V., Kuskov, I.V., Zosimov, A.V., Antipenko, E.E., and Lunin, V.V., J. Anal. Chem., 2003, vol. 58, no. 5, p. 439.
Yukichi, Y., Iwao, T., Masakazu, K., and Takashi, U., Bull. Chem. Soc. Jpn., 1974, vol. 47, no. 11, p. 2787.
Cartledge, G.H. and Ericks, W.P., J. Am. Chem. Soc., 1936, vol. 58, no. 10, p. 2069.
Takashi, U., Iwao, T., and Yukichi, Y., Bull. Chem. Soc. Jpn., 1975, vol. 48, no. 10, p. 2809.
Martell, A.E. and Smith, R.M., Critical Stability Constants, vol. 3. Other Organic Ligands, Boston: Springer, 1977.
Kettler, R.M., Palmer, D.A., and Wesolowski, D.J., J. Solution Chem., 1991, vol. 20, no. 9, p. 905.
Adler, S.J. and Noyes, R.M., J. Am. Chem. Soc., 1955, vol. 77, no. 8, p. 2036.
Tyupalo, N.F. and Yakobi, B.A., Zh. Neorg. Khim., 1980, vol. 25, no. 6, p. 1557.
Jacobsen, F., Holcman, J., and Sehested, K., Int. J. Chem. Kinet., 1998, vol. 30, no. 3, p. 207.
Levanov, A.V., Kuskov, I.V., Antipenko, E.E., and Lunin, V.V., Russ. J. Phys. Chem. A, 2008, vol. 82, no. 7, p. 1126.
Levanov, A.V., Isaikina, O.Y., and Lunin, V.V., Russ. J. Phys. Chem. A, 2020, vol. 94, no. 1, p. 81.
Funding
This work was carried out within the framework of the state assignment “Surface Physicochemistry, Adsorption, and Catalysis.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
About this article
Cite this article
Levanov, A.V., Isaikina, O.Y. & Gryaznov, R.A. Catalytic Ozonation of Oxalic Acid in Aqueous Solution in the Presence of Manganese Ions. Kinet Catal 63, 180–187 (2022). https://doi.org/10.1134/S0023158422020069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158422020069



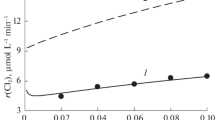

 ) KMnO4 or (
) KMnO4 or ( ) MnSO4, and the line illustrates the model calculation.
) MnSO4, and the line illustrates the model calculation. 
 ) KMnO4 or (
) KMnO4 or ( ) MnSO4, and the line illustrates the model calculation.
) MnSO4, and the line illustrates the model calculation. 
 ) KMnO4 or (
) KMnO4 or ( ) MnSO4, and the line illustrates the model calculation.
) MnSO4, and the line illustrates the model calculation. 


