Abstract
In this work CaO-based catalysts were found to be efficient heterogeneous catalysts for the methylation of phenol with dimethyl carbonate (DMC) in a closed high pressure reactor. The optimization experiments have been carried out to obtain best phenol conversion and the results showed that CaO catalyst modified with KCl had the best catalytic performance. When the reaction was carried out at 200°C, with phenol to dimethyl carbonate molar ratio of 1 : 2, 15% KCl/CaO catalyst dosage of 3%, reaction time 9 h, 100% conversion of phenol and 95% selectivity towards anisole have been achieved. The structure and properties of the materials were thoroughly characterized by Fourier transform infrared spectrometry (FTIR), scanning electron microscopy (SEM), and Brunauer–Emmett–Teller (BET). The close correlation was found between surface basicity of the catalysts and their catalytic performance for phenol conversion and anisole selectivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Anisole is an important organic synthetic intermediate that is widely used in industry for synthesis of dyes, pesticides, pharmaceuticals, etc. [1–3], which are conventionally produced by O-methylation of the corresponding phenols with methyl halides or dimethyl sulfate. However, these methods require not only harmful reagents but also a stoichiometric amount of a strong base to neutralize acid by-product. Instead, the O-methylation of phenols can also be conducted with methanol in the presence of a strong acid catalyst or a zeolite at high temperature. In most of the cases, total conversion of phenols was difficult to attain and the C-methylation always occurred in competition with the O-methylation [4–12]. Dimethyl carbonate (DMC), an environmentally friendly methylation agent, is a substitute for traditional methylation agents such as methyl halides (CH3X, X = I, Br, Cl) or dimethyl sulfate [13]. In recent decades, an efficient, convenient, and green method has been developed for O-methylation of phenol with DMC. DMC is a well-known nontoxic reagent that has been used for many green applications. In fact, DMC gives only methanol and carbon dioxide as byproducts when used as a methylation agent [14]. With the enhancement of awareness that people need environmental protection, more and more research on this technology has been done.
Phase transfer catalysts, organic base catalysts, and zeolite catalysts [15] are efficient catalysts for the reaction of DMC with phenol to synthesize anisole. Thus, the synthesis of anisole by O-methylation of phenol with DMC had been extensively investigated and high catalytic performance was achieved compared to phase transfer and organic catalysts. However, the reactions generally carried out homogeneously cannot avoid the drawbacks due to a poor catalyst recovery and complicated product separation. For zeolites, higher reaction temperature (250–300°С) leads to production of more side products of phenyl ring C-alkylation. Although various heterogeneous catalysts have been investigated, less effort has been made in the methylation of phenol. Hence, there is a crucial need to exploit highly efficient heterogeneous catalytic materials for this reaction [16].
In this work, CaO loaded with alkali metal chloride were adopted as catalysts for the O-methylation of phenol with DMC and the effect of the reaction conditions on the reactivity was also been investigated in detail. The reaction routes to the major products and the catalytic mechanism were suggested.
EXPERIMENTAL
Catalysts Preparation
In this experiment, a series of CaO-based catalysts was prepared by an incipient wetness impregnation method of CaO with an aqueous solution of metal chloride. In all cases, 6 g of ~100–200 mesh commercial CaO powder was preheated at 900°C for 6 h to remove physically absorbed water and carbon dioxide, and put it into a drying dish for use. After stirring at room temperature for 10 h of CaO particles with various metal chloride solutions, the precipitate was dried in an oven overnight at 105°C. Prior to the reaction, all the catalysts were calcined in air at desired temperatures for 6 h. All the catalysts were named as x% YCl/CaO, where x is the loading amount of YCl.
Characterization of Catalyst
The morphology of the prepared catalysts was elucidated using a scanning electron microscope (SEM, JSM-6390A, JEOL, Japan) with 20.0 kV of an accelerating voltage. The phase structures of the calcined catalysts were analyzed using an X-ray diffraction device (JDX-3530, JEOL, Japan), with an X‑ray tube having copper (Cu) as a target and released Kα radiation when accelerated at 40 mA and 40 kV, arranged at 5°–80° with a scanning speed of 2°/min. Surface area and pore property measurements were performed by N2 physisorption using a instrument ASAP 2010 (Micromeritics, USA) at 77 K. The surface areas were calculated using the BET equation in the pressure of range P/P0 = 0.02–0.2, and the pore size distribution was calculated using the Barrett–Joyner–Halenda (BJH) method. The base-strength distribution of the catalysts was measured using a temperature-programmed desorption (TPD) of CO2 with an instrument ChemiSorb2750 (Micromeritics, USA). The catalyst sample was pretreated in Ar flow at 300°C for 1 h, and cooled to 50°C for 3 h in carbon dioxide. Then, the temperature of the sample was increased from 50 to 800°C at a ramp rate of 10°C min under He atmosphere, and the desorbed carbon dioxide was detected using a thermal conductivity detector. The infrared spectra of the samples were measured in the form of KBr (ratio 1 : 100) powder pellets on a Fourier transform infrared (FTIR) spectrometer Nicolet 5700 (Thermo Electron Co., USA) under ambient conditions.
Methylation Reaction of Phenol with DMC
The methylation reaction of phenol with DMC was carried out in a stainless steel reactor. Phenol, DMC and the catalyst were added into the autoclave (100 mL) in proportion and heated to the settled reaction temperature. After obtaining homogeneous mixture under magnetic stirring for the desired time, the catalysts were separated by centrifugation and the supernatant was collected for analysis by gas chromatography. The yield of anisole was quantitatively determined with acetophenone as internal standard. The products were quantitatively analyzed by gas chromatograph Agilent 6890 (Agilent, USA) equipped with a PONA capillary column (50 m × 0.2 mm × 0.5 μm) and a flame ionization detector (FID). The conversion and selectivity were calculated based on the phenol [17].
RESULTS AND DISCUSSION
Screening of Catalysts
The catalyst screening experiments were carried on fresh CaO and various CaO supported catalysts with 15% amount of active components, i.e., LiCl, NaCl and KCl (Table 1) summarizes the effect of the active metal chloride on the catalytic performance of CaO-supported catalysts (phenol conversation, methylation reaction selectivity and anisole yield) with a 1 : 2 molar ratio of phenol to DMC at 180°C. From the results it can be found that after 10 h of reaction all CaO-based samples exhibited favorable activity after modifying with chloride salts, and the highest catalytic activity, 36.8% conversation of phenol and 46.8% selectivity of anisole, was obtained on KCl/CaO. These results can be partially related to the presence of K sites of the catalyst surface which greatly enhance the adsorption of phenol as suggested in previous research [18]. Considering the higher activity of KCl/CaO, the following optimization experiments were carried out with this catalyst.
Physical Adsorption Characterization
In order to explain the high catalytic activity of CaO catalyst modified with alkali chloride, N2 adsorption-desorption experiments were carried out to obtain detailed pore size distribution information. Figure 1 summarized N2 adsorption–desorption isotherms information of all samples. It was observed that except for LiCl/CaO, the nitrogen adsorption and desorption curves of the samples showed hysteresis loops within the relative pressure range of ~0.4–0.8, that is, the addition of KCl or NaCl greatly enhance the pore size of CaO and the pore structure of CaO modifying with KCl showed the largest hysteresis loop indicating its mesoporous structure. Furthermore, the relative pressure range (P/P0 ≈ 0.4–1.0) of KCl/CaO and NaCl/CaO increased, and there was no adsorption limit in the higher pressure range inferred that there are mainly two types of pore structures [19].
The textural properties of the sample are listed in Table 2. It can be seen that catalyst modifying with metal chloride resulted in considerable decrease in the specific surface areas due to the second calcination under high temperature. The pore size distribution of the supported catalysts was mainly between 2 and 50 nm indicating typical mesoporous properties. The increase in pore volume of the catalysts is of greatly importance in heterogeneous reaction to improve the diffusion limit of the reaction molecules and make it easier for the reactants to enter the catalyst and contact with active sites. At the same time, the internal pore structure of the catalysts also affects the number of alkaline sites. It can be concluded from the pore structure analysis results that the pore structure of solid catalyst is one of important factor affecting its catalytic activity as well as its selectivity.
Fourier Transform Infrared (FTIR)
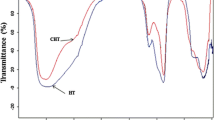
The FTIR spectra of CaO and CaO modified with different alkali metal chlorides are shown in Fig. 2. The band at 3641 cm–1 can be ascribed to the O–H symmetric stretching mode of the hydroxyl group and the broad band in the region of 3200–3600 cm–1 can be assigned to asymmetric stretching vibrations of surface hydroxyl groups indicating that the moisture effect cannot be avoided during catalyst preparation process. The peaks observed at 1380 and 876 cm–1 are due to \({\text{CO}}_{3}^{{2 - }}\) vibration. The formation of –OH and \({\text{CO}}_{3}^{{2 - }}\) indicates that the erosion by CO2 and water during the catalyst preparation process was intensified especially using the initial impregnation method. Compared with other samples, relatively low intensity of the –OH and \({\text{CO}}_{3}^{{2 - }}\) vibration bands was found for KCl/CaO. This indicates that the presence of KCl improves the stability of CaO in air to a certain extent as well as its high catalytic performance as mentioned above.
The Effect of Catalyst Amount on the Methylation Reaction
The effect of the KCl/CaO amount on the methylation reaction of phenol with DMC was first studied with a 1 : 2 molar ratio of phenol to DMC at 180°C and the results are listed in Table 3. Under the reaction condition used herein, only two products of anisole (AN) and methylphenyl carbonate (MPC) were detected. When 1.5% catalyst was introduced, the phenol conversion and methylation selectivity were 36.8 and 46.8%, respectively. With increasing amount of the catalyst to 3%, the phenol conversion increased to 64.9% with high anisole selectivity of 83.2%, indicating that more basic sites improved the conversion of phenol to the target product anisole. However, further increase in the catalyst amount caused the phenol conversion to decrease, but the high anisole selectivity still remained. Thus, it seemed that some combination of base might lead to a high catalytic performance.
Effect of Reaction Temperature on Catalytic Performance of KCl/CaO
The effect of reaction temperature on KCl/CaO catalyzed methylation of phenol with DMC is shown in Table 4. The reaction was conducted at temperatures from 160 to 220°C with 3% KCl/CaO and molar ratio of phenol to DMC of 1 : 2. It can be seen that as the reaction of methylation of DMC with phenol is thermodynamically unfavorable and endothermic, it should proceed at high temperature to increase the yields for anisole. The methylation capacity of DMC is enhanced with an increase in the reaction temperature up to 200°C. Further increase in the reaction temperature does not raise catalytic activity noticeably. Therefore, 200°C is taken to be the optimal reaction temperature for the purpose at hand. The selectivity of anisole increased with increasing reaction temperature, and in particular, the formation of anisole was promoted most significantly at high temperature over 180°C. The results are similar to previous work which indicated that a high temperature favored the O-methylation of phenol [20].
Effect of KCl/CaO Calcination Temperature on Catalytic Performance
The effect of KCl/CaO calcination temperature on the methylation reaction of phenol with DMC is shown in Table 5. The reaction was conducted under the same conditions as before. Aiming to increase the activity of KCl/CaO, the calcination temperature was varied from 400 to 800°C. The catalytic performance enhanced with increasing calcination temperature from 400 to 500°C, and conversion reached 100% at 500°C, anisole being the main product. Possible reasons for the effect of KCl/CaO calcination temperature on its catalytic performance will be discussed in detail based on the sample characterizations in our following experiments.
Physical Adsorption Characterization
The textural properties of the catalyst sample are listed in Table 6. It can be seen that high calcination temperature caused a great decrease of the surface area along with narrowing the pore size. The largest specific surface area (41.23 m2/g) and pore volume (0.2097 × 10–2 cm3/g) were obtained over KCl/CaO calcined at 500°C. This treatment provided efficient channels for the rapid diffusion of phenol and DMC so as to reach almost 100% conversion of phenol and selectivity of anisole as shown in Table 5.
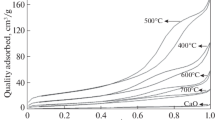
Nitrogen adsorption–desorption experiments were performed to obtain detailed information on the pore size distribution of CaO modified with KCl calcined at different temperatures. Figure 3 demostrates that the N2 adsorption–desorption isotherms of KCl/CaO calcined at 500°C showed apparently different patterns from other samples indicating that the porosity of CaO has greatly changed after modification. The clear hysteresis loop for the sample calcined at 500°C at a high relative pressure range indicates the formation of extra (meso) porosity during calcination process, which was consistent with its large pore volume [21] as shown in Table 6. However, the samples calcined at higher temperatures showed a poor hysteresis loop as a result of the pore structure collapse. As suggested previously, the pore diameter plays an important role in the methylation reaction. The highest catalytic performance was obtained over 500°C of KCl/CaO which has the largest average pore diameter [22].
Fourier Transform Infrared (FTIR) Spectroscopy
Figure 4 is an infrared characterization of KCl/CaO prepared by calcination at different temperatures. In all spectra, there are obvious \({\text{CO}}_{3}^{{2 - }}\) stretching vibration peaks at 1450 and 876 cm–1 mainly due to the chemical adsorption of CO2 from air. They gradually weakened with increasing calcination temperature. In addition, the intensive peaks at 3652 and 3440 cm–1 from the stretching vibration of surface hydroxyl groups indicated that KCl/CaO is inevitably corroded by air during the preparation process [23].
The Effect of KCl/CaO Loading on Catalytic Performance of Methylation
Under the same reaction conditions given above, the activity of calcined KCl/CaO (at 500°C for 6 h) under different KCl loadings were investigated and shown in Table 7. When the KCl loading amount increased from 5 to 15%, phenol conversation increased from 74.9 to 100% and then somewhat decreased at the KCl content above 15%. Anisol selectivity showed the same tendency as the phenol conversion: the highest selectivity (almost 100%) was reached at the phenol conversion of 100%. On the contrary, the selectivity to methyphenol was higher at lower KCl contents. This indicated that a low KCl content was advantageous for C-methylation conversion of phenol, while a high content of KCl favored the conversion of phenol by O-methylation, illustrating the importance of KCl on the formation of anisole. To give a reasonable explanation for the effect of KCl loading on the performance of KCl/CaO, further characterizations were carried out using SEM, XRD, CO2-TPD and FTIR.
Scanning Electron Microscopy Characterization (SEM)
Figure 5 depicts scanning electron micrographs of CaO and KCl/CaO samples. It can be seen that the surface of CaO before loading mainly appeared in a spherical aggregate state with smaller particle size, which gradually increased with the introducing of KCl, and it appeared as relatively dispersed flake particles. It was illustrated previously that the inorganic salt addition could be used to adjust the pore diameter of mesoporous materials, and could result in the transformation of the mesophase, which is directly dependent on the concentration and nature of salt added [24].
Fourier Transform Infrared (FTIR)
Figure 6 shows the infrared spectra of KCl/CaO with different KCl loadings. As suggested before, the characteristic stretching vibration peak of the physical adsorption of water and CO2 still could be found over all samples indicating the formation of –OH and \({\text{CO}}_{3}^{{2 - }}\) mainly due to the effect of the moisture and carbon dioxide in air on the catalyst during preparation and storage. The results showed that the intensity of the –OH and \({\text{CO}}_{3}^{{2 - }}\) vibration peaks were significantly weakened for 10 and 15% KCl/CaO compared to unmodified CaO, indicating that the presence of KCl improved the stability of CaO in air as well as its catalytic performance in methylation.
Chemical Adsorption Characterization (CO2-TPD)
Figure 7 shows CO2-TPD profiles for CaO and all modified CaO with various KCl amounts recorded from 25 to 800°C. It is worth noting that only one large broad desorption peak was found for all the samples in the temperature range of 660–800°C attributed to the strong basic sites of O2– anions. Furthermore, the desorption peaks of all KCl/CaO samples moved to a higher temperature compared to that of CaO (desorption peak at 664.4°C); i.e., the incorporation of potassium increased the basic strength. However, excessive KCl will cause the alkaline sites on the surface of CaO to be covered so as to the small number of basic sites was reflected by the small desorption peak area. Thus, the relationship between the basic properties and the catalytic activity for this base-catalyzed methylation reaction was found.
X-ray Diffraction Characterization (XRD)
The XRD patterns of the CaO samples modified with different amounts of KCl are shown in Fig. 8. Addition of KCl weakened the CaO diffraction peak, but the diffraction peak intensity of CaCO3 and Ca(OH)2 increased with increasing KCl loading. Obvious characteristic diffraction peaks of Ca(OH)2 for all CaO-based samples modified with KCl indicated that the surface hydration of CaO particles could not be avoided as already mentioned above. For CaO modified with 15% KCl, diffraction peak intensity of CaO reached a maximum value indicating a higher dispersion of CaO particles in line with the uniform morphology as suggested by SEM results.
The Possible Mechanism of Methylation of Phenol with DMC
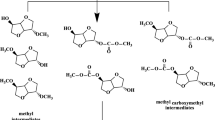
The possible reaction mechanism is shown in Fig. 9. It can be deduced that phenol was activated by the KCl/CaO catalyst to phenol salt (PhO.A) at first. The oxygen anion of the phenol salt is a strong nucleophilic reagent, nucleophilic substitution reaction was carried by phenoxy anions of phenol salts which attacked the methyl carbon of DMC and then formed a six-membered ring, which was rearranged to anisole and methyl carbonate. Methyl carbonate was further decomposed into carbon dioxide and methanol which makes the reaction more difficult and takes a long time [25].
CONCLUSIONS
In summary, a series of CaO supported alkali metal chloride catalysts were prepared and showed the excellent catalytic activity and selectivity for the methylation reaction between phenol and DMC. Among the obtained catalysts, an appropriate amount of KCl (15%) supported on CaO gave the best results to produce anisole. And the phenol conversion and the selectivity to anisole were found to increase in parallel with the amounts of the KCl and strong basic sites. When the reaction was carried out with a phenol to DMC molar ratio of 1 : 2 at 473 K (200°C) for 8 h, the phenol conversion could reach 100 with 100% selectivity towards anisole over 15% KCl/CaO. Furthermore, the mesoporous structure promoted the selectivity by improving O-methlytion reaction. Thus, typical environmentally hazardous homogeneous catalysts such as alkaline solutions could be replaced with KCl/CaO catalysts, which are able to significantly improve the industrial process for the production of anisole from phenol.
REFERENCES
Perego, C. and Ingallina, P., Green Chem., 2004, vol. 56, p. 274.
Vijayaraj, M. and Gopinath, C.S., Appl. Catal., A, 2007, vol. 320, p. 64.
Byun, E., Hong, B., Castro, K., Lim, M., and Rhee, H., J. Org. Chem., 2007, vol. 72, p. 9815.
Oae, S. and Kiritani, R.B., Bull. Chem. Soc. Jpn., 1966, vol. 39, p. 611.
Santacesaria, E., Grasso, D., Gelosa, D., and Carra, S., Appl. Catal., A, 1990, vol. 64, p. 83.
Porchet, S., Su S., Doepper, R., and Renken, A., Chem. Eng. Technol., 1994, vol. 17, p. 108.
Porchet, S., Kiwi-Minsker, L.K., Doepper, R., and Renken, A., Chem. Eng. Sci., 1996, vol. 51, p. 2933.
Bautista, F.M., Campelo, J.M., Garcia, A., Luna, D., Marinas, J.M., Romero, A.A., and Urbano, M.R., Catal. Lett., 1997, vol. 62, p. 47.
Samolada, M.C., Grigoriadou, E., Kiparissides, Z., and Vasalos, I.A., J. Catal., 1995, vol. 152, p. 52.
Lee, S.C., Lee, S.W., Kim, K.S., Lee, T.J., Kim, D.H., and Chang Kim, J., J. Catal. Today, 1998, vol. 44, p. 253.
Bal, R., Chaudhari, K., and Sivasanker, S., Catal. Lett., 2000, vol. 70, p. 75.
Izotov, A.I., Kilman, G.V., and Shalayev, R.V., Russ. J. Phys. Chem. B, 2019, vol. 13, p. 1169.
Yan, H.D., Zeng, L.F., Xie, Y.Q., Cui, Y., Ye, L., and Tu, S., Rev. Chem. Intermed., 2016, vol. 42, p. 5951.
Aricò, F. and Tundo, P., Russ. Chem. Rev., 2010, vol. 79, p. 479.
Ouk, S., Thiebaud, S., Borredon, E., and Le Gars, P., Appl. Catal., A, 2003, vol. 241, p. 227.
Mareev, E.I., Aleshkevich, V.A., Potemkin, F.V., Minaev, N.V., and Gordienko, V.M., Russ. J. Phys. Chem. B, 2019, vol. 13, p. 1214.
Xiao, Z.L., Yang, H., Zhang, H., Chen, T., and Wang, G.G., Chem. Pap., 2018, vol. 72, p. 2347.
Kosyakov, D.S., Ul’yanovskii, N.V., Ivakhnov, A.D., and Pikovskoi, I.I., Russ. J. Phys. Chem. B, 2019, vol. 13, p. 1103.
Pan, D.H., Dian-Chu, L.I., and Jing-Hong, M.A., Acta Petrol. Sinica, 2005, vol. 8031, p. 256.
Fedyaeva, O.N. and Vostrikov, A.A., Russ. J. Phys. Chem. B, 2019, vol. 13, p. 1071.
Petrov, L.V. and Solyanikov, V.M., Russ. J. Phys. Chem. B, 2020, vol. 14, p. 29.
Balasubramanian, V.V., Pandurangan, A., Palanichamy, M., and Murugesan, V., Indian. Chem. Technol., 2000, vol. 7, p. 149.
Chen, B., Zhao, N., and Wei, W., Pet. Calt., 2009, vol. 38, p. 897.
Maleki, H. and Kazemeini, M., J. Fuel Chem. Technol., 2017, vol. 45, p. 442.
Chen, B., Zhao, N., and Wei, W., et al., J. Fuel Chem. Technol., 2011, vol. 39, p. 144.
Funding
This work was financially supported by National Science Foundation of China (21808182), State Key Laboratory of Petroleum Pollution, Scientific Research Program Funded by Shaanxi Provincial Education Department (17JS114), Xi’an science and technology project (201805038YD16CG22(3)) and the Open Project Program of State Key Laboratory of Petroleum Pollution Control, CNPC Research Institute of Safety and Environmental Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Abbreviations and designations: DMC, dimethyl carbonate; FTIR, Fourier transform infrared spectrometry; SEM, scanning electron microscopy; XRD, X-ray diffraction; CO2-TPD, temperature-programmed desorption of CO2; BJH, Barrett–Joyner–Halenda method; BET, Brunauer–Emmett–Teller method; AN, anisole; MPC, methylphenyl carbonate.
Rights and permissions
About this article
Cite this article
Zhang, Z., Xu, Z., Li, S. et al. Selective O-Methylation of Phenol with Dimethyl Carbonate over Catalysts Supported on CaO. Kinet Catal 62, 496–506 (2021). https://doi.org/10.1134/S0023158421040145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158421040145