Abstract
The kinetics of interaction between glutathione (GSH) and unsaturated phenol resveratrol (RVT) in deionized water in the presence of hydrogen peroxide (H2O2) is studied. At a physiological concentration (0.1–10 mM), GSH containing two carboxyl groups forms acidic solutions (pH of 3–4); the GSH molecules are associated into dimers. Under these conditions, GSH is quite slowly oxidized by atmospheric oxygen, and the reaction between GSH and H2O2 is accompanied by the formation of radicals. The thiyl radical initiation rate (Wi) is a few fractions of a percent of the GSH consumption rate; however, it is sufficient to initiate a thiol–ene chain reaction between GSH and RVT. Using the experimental data on the kinetics and the product composition and the published data on reactions of GSH with H2O2 and thiyl radicals, a kinetic model of the complex interaction between GSH and RVT in the presence of H2O2 in an aqueous medium at 37°C is proposed. The model includes 19 quasi-elementary reactions with respective rate constants, in particular, the formation of intermediate GSH–H2O2 and GSH–GSH complexes, the formation of radicals, and their subsequent transformations into final products in reactions with RVT and GSH. A computer simulation based on the developed model adequately describes the features of the process kinetics in a wide reactant concentration range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Glutathione (GSH) is a natural endogenous thiol contained in biological tissues and fluids in high concentrations (0.1–10 mM), which are a few orders of magnitude higher than the concentrations of other components of the antioxidant system. It is believed that GSH regulates protein functions and gene expression, reacts with hydroxyl and peroxyl radicals, reduces hydroperoxides and disulfide bonds, and hinders protein oxidation [1–3]. There are reports on significant changes in the GSH content during the development of many pathologies, in particular, Alzheimer’s, Parkinson’s, cardiovascular, and oncological diseases [4–9].
Earlier, the mechanism of the reaction between GSH and H2O2 in a bidistilled deionized water medium was studied in detail [10–14]. It was found that the reaction between GSH and H2O2 is accompanied by the formation of radicals [10, 11]. Using the inhibitor method and employing an original radical acceptor [10, 15], it was shown that, in deionized water, the formation of radicals in reaction with H2O2 is also observed in the case of other thiols, namely, cysteine, homocysteine, and acetylcysteine. The authors of [10], using their own and published data, constructed a kinetic model of interaction between GSH and H2O2 including 13 quasi-elementary reactions with respective rate constants, which adequately described the kinetic curves of GSH consumption and radical initiation (radical acceptor consumption). The authors of [16], using the spin trap method and employing 5,5-dimethyl-1-pyrroline-N-oxide, showed that the interaction between GSH and H2O2 really leads to the formation of thiyl radicals. The radical yield is low; however, even this amount of radicals is sufficient to initiate chain processes. It was found [13, 14] that, in the presence of H2O2 in aqueous solutions, thiol–ene chain reactions of GSH with unsaturated phenols resveratrol (RVT) and caffeic acid are initiated. The reactions of thiols with olefins (thiol–ene reactions, alkene hydrothiolation) to form thioesters have been known for a long time, namely, since 1905 [17]. However, in recent decades, considerable attention has been paid to these reactions owing to the possibility of selectively and stereoselectively synthesizing various compounds in polymer and medicinal chemistry [18–20]. Resveratrol and caffeic acid are plant polyphenols, lignin biosynthesis metabolites; they contain an unsaturated bond in the side substituents of the aromatic framework. Recently, these phenols, in particular RVT (3,5,4'-trihydroxystilbene), have attracted the attention of physicians and biochemists owing to the so-called “French paradox,” i.e., an unusually low level of cardiovascular and oncological diseases despite a high-calorie diet with an abundance of fat that is observed in some regions of France against the background of regular consumption of red wine [21, 22]. Owing to the presence of an unsaturated bond conjugated with two phenolic moieties (Scheme 1), RVT can exist in the trans- and cis-form and intensively react with thiyl radicals, which, at high rate constants (~105 M−1 s−1), undergo reversible addition to double bonds and catalyze the cis–trans isomerization of olefins [23, 24].
In this work, the kinetics of interaction between GSH and RVT in the presence of H2O2 is experimentally studied; the composition of the products formed in reactions of GSH with H2O2 and RVT is studied by mass spectrometry (positive-ion electrospray ionization mass spectrometry). Taking into account the derived data on the thiol–ene reaction between GSH and RVT and the published data on reactions of GSH, H2O2, and thiyl radicals, a refined kinetic model of the complex interaction between GSH and H2O2 and the thiol–ene reaction with RVT (in an aqueous medium at 37°C) is proposed; the model adequately describes the features of the process kinetics in a wide reactant concentration range.

Scheme 1 . Structural formulas of GSH and RVT.
EXPERIMENTAL
Glutathione, Ellman’s reagent (5,5'-dithiobis(2-nitrobenzoic acid) (DTNB)), hydrogen peroxide Н2О2 (PanReac AppliChem), and trans-RVT (ABCR GmbH) were used without further purification.
Deionized water was used as the reaction medium.
The base solution of RVT (13.3 mM) was prepared in ethanol (Medkhimprom), which was added to the reaction mixture. The H2O2 concentration (in the absence of GSH) was controlled by iodometry. The GSH concentration was determined spectrophotometrically using the Ellman’s reagent at λmax = 412 nm and ε = 0.14 × 105 M−1 cm−1 [25, 26].
The thiol–ene reaction between GSH and RVT was run at 37°С directly in a temperature-controlled cell of an SF-2000 spectrophotometer (OOO OKB Spectr, Russia), in which the RVT consumption was recorded at ε = 0.3 × 105 M−1 cm−1 and λmax = 304–308 nm, and in a temperature-controlled glass cell equipped with sampling and air bubbling devices. During reaction, 90-μL aliquots were taken from the reaction vessel to analyze RVT and GSH. The aliquots were added to 3 mL of deionized water and a phosphate buffered saline (PBS, pH of 7.4) containing 0.3 mM DTNB, respectively, to record ultraviolet spectra.
Glutathione containing two carboxyl groups forms acidic solutions (pH of 3–4) at a physiological concentration (0.1–10 mM). Significant differences in the kinetics and mechanism of the reaction between GSH and H2O2 in deionized water and phosphate buffer systems with pH ≥ 7, which are commonly used in biochemical studies, were found in [27]. Therefore, in each experiment, the pH of the solutions was measured using a pH-410 pH-meter/millivoltmeter (Akvilon, Russia). The error in pH measurements was ±0.02. The error in measuring the GSH and RVT consumption rates did not exceed 15%.
The molecular products of the reaction were studied at the Center for collective use “New Materials and Technologies” of Emanuel Institute of Biochemical Physics of the Russian Academy of Sciences on an LTQ FT Ultra tandem mass spectrometer (Thermo Finnigan, Germany) by electrospray ionization mass spectrometry in the positive-ion measuring mode. Immediately before placing a sample into the mass spectrometer, the sample was diluted 20-fold with a 50% acetonitrile solution with the addition of 0.1% formic acid.
The computer simulation of the kinetic curves of reactant consumption in the reaction between GSH and RVT in the presence of H2O2 and the optimization of the rate constants of 19 quasi-elementary reactions constituting the kinetic model of the process were conducted as described in [28].
RESULTS AND DISCUSSION
Kinetic Features of the Thiol–Ene Reaction between GSH and RVT in the Presence of Hydrogen Peroxide in Deionized Water
It was noted above that the GSH concentration in biological tissues and fluids is a few orders of magnitude higher than the micromolar concentration of other components of the antioxidant system. Therefore, in experiments, [GSH] was typically varied in a range of 0.1–10 mM, and [RVT] were on the order of 1–100 μM. Figure 1 shows the kinetic curves of RVT consumption (curve 1) in the absence and (curves 2–5) in the presence of H2O2 at different GSH concentrations. It is evident that the consumption of RVT, which is an effective radical acceptor, is observed only in the case of the simultaneous presence of GSH and H2O2 (curves 3–5). It should be noted that the introduction of GSH into the reaction medium leads to a decrease in pH (Table 1).
Kinetic curves of consumption of 0.03 mM RVT in reaction with GSH (1) in the absence and (2–5) in the presence of 4.55 mM H2O2; the GSH concentration (mM): (1) 25 (RVT concentration of 0.033 mM), (2) 0, (3) 2.5, (4) 5, and (5) 10. The symbols denote the experimental data; the solid lines stand for the data calculated in terms of the kinetic model (Table 2).
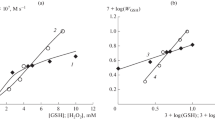
Figure 2 suggests that the initial RVT consumption rate (WRVT) increases linearly with an increase in the initial RVT concentration. Earlier, the authors of [10] obtained empirical dependences for GSH consumption rate (WGSH) and radical initiation rate (Wi), which was measured by the inhibitor method, during the reaction between GSH and H2O2:
The RVT consumption rate (WRVT), in common with WGSH [10], nonlinearly depends on GSH and H2O2 concentrations. Table 1 lists WRVT values experimentally measured at different GSH concentrations in the presence of 4.55 mM H2O2 and radical initiation rates (*Wi) calculated using Eq. (2). It is noteworthy that the rate values cut off by the linear WRVT–[RVT] dependences on the axis of ordinates (Fig. 2) are almost identical (within the error limits) to the calculated *Wi values for respective GSH concentrations. According to Table 1, the chain length in RVT consumption (WRVT/Wi) is small—on the order of two units,—and the radical yield is lower than 1% (Wi/WGSH < 0.01).
Resveratrol consumption rate is satisfactorily described by Eq. (3) for chain reactions of oxidation and polymerization with quadratic chain termination at chain-carrying radicals [29]. It was assumed [13, 14] that, in the chain reaction of unsaturated phenols with GSH in the presence of H2O2, termination occurs at GS• thiyl radicals and the rate-limiting step is the reaction between the radical and RVT:
Here, the а ≅ 3.5 M−0.5 s−0.5 parameter is similar to the ratio between the rate constants of the chain propagation (kp) and chain termination reactions (kt):
Product Analysis
The molecular products of the reaction were studied by electrospray ionization mass spectrometry in the positive-ion measuring mode. Figure 3a shows a mass spectrum of the original GSH sample, which exhibits, along with the МН+ 308.09 molecular ion, М'Н+ 615.17 ions, which indicate the presence of fairly stable GSH–GSH dimers in the sample. It was reported [30] that studies of the mass spectra of GSH by negative-ion electrospray ionization reveal the presence of dimer ions, along with GSH ions, in an aqueous solution, whereas no dimers are detected in a PBS (0.1 M, pH ~ 7). Apparently, GSH ions that are similarly—negatively—charged owing to the dissociation of carboxyl groups do not form dimers at pH ≥ 7.
According to Fig. 3b, the main product of GSH oxidation in reaction with H2O2 is the respective GSSG disulfide (M''Н+ 613.16). The reaction occurs in accordance with the following well-known and repeatedly confirmed stoichiometric equation [30–34]:
Figure 3c shows a mass spectrum of products formed in a reaction mixture of 2.3 mM GSH, 1.3 mM RVT, and 3.2 mM H2O2 in initially deionized water; it is evident that the main product is GSSG disulfide (MН+ 613.16). The formation of an МН+ 568.16 product, along with the formation of GSSG, is observed; the mass of the product corresponds to hydroperoxide (PO2H), which can result from the sequential addition of the GS• thiyl radical and oxygen to RVT:
Kinetic Model of Interaction between GSH and RVT
The kinetics of interaction between GSH and RVT in the presence of H2O2 was analyzed by computer simulation as described in [28]. Earlier, a kinetic model of interaction between GSH and H2O2, which included 13 quasi-elementary reactions, was described [10]. Taking into account additional experimental data and refined published data, ten reactions were left to describe the interaction between GSH and H2O2 (Table 2, reactions (I)–(X)).
Since the authors of a set of studies [35–38] provide quite convincing results of spectroscopic (ultraviolet and infrared) studies and theoretical analysis [36] of the formation of GSH–H2O2 complexes not only in buffer solutions with physiological pH, but also in pure water [37], which has a pH of 2 upon the addition of GSH, we preserved reactions (I)–(III), which describe the formation of complex K (GSH–H2O2) and the complex oxidation to GSSG disulfide, in the kinetic model, although the МН+ 342 ion corresponding to the complex is not detected in the mass spectra of the reaction products (Fig. 3b).
It is noteworthy that the GSH consumption rate of 2 × 10−3 M/s that was measured by the time-resolved Raman spectroscopy method at a reactant concentration of 1 M in [37] is almost identical to the WGSH rate of 1.8 × 10−3 M/s that was calculated by Eq. (1).
It was shown [27] that, in phosphate buffer systems at pH ≥ 7, the GSH oxidation by atmospheric oxygen is enhanced, while the radical initiation rate in the reaction between GSH and Н2О2 abruptly decreases compared with Wi in deionized water. At pH ≥ 7, GSH dimers are not formed. Therefore, in constructing the model, it was assumed that the formation of radicals occurs mostly in reaction (VII) during the interaction between the GSH–GSH dimer (C, Table 2) and H2O2. Reactions (VII) and (VIII), which supply radicals, have hardly any effect on the GSH consumption rate (WGSH). The –SH thiol group –SH in complexes K [35] and C is determined by the Ellman’s reagent as in free GSH. The k10 value of 109 M−1 s−1 is known for a rapid recombination of thiyl radicals [34].
Reactions (XI)–(XVI) occur in the case of RVT additives and, together with other reactions, describe the kinetic curves of RVT consumption. It is known that thiyl radicals with high rate constants (~105 M−1 s−1) undergo reversible addition to –C=C– double bonds [23, 40]; therefore, reactions (XI) and (XII) were introduced into the model. The P• alkyl radical resulting from the addition of GS• to RVT can react with GSH (k13 ≈ 105–106 M−1 s−1 [23, 40]) or oxygen, because the experiments were conducted under aerobic conditions (k14 ≈ 109–1010 M−1 s−1 [41]). Table 3 lists quasi-stationary radical concentrations calculated in terms of the model; it is evident that, in the presence of О2, \({\text{PO}}_{2}^{\bullet }\) peroxyl radicals are dominant and the content of molecular products of the addition of GS• radicals to RVT increases. The determining role of О2 in the kinetics of the addition of thiyl radicals to olefins was reported and analyzed by the authors of [41, 42], who studied it by flash photolysis.
Figure 4 shows experimentally repeated and reproducible kinetic curves of consumption of GSH and RVT taken in concentrations comparable in scale. Against expectations, in the presence of RVT, the GSH consumption rate decreases, rather than increases owing to the additional consumption in the chain reaction with RVT. To provide the occurrence of this effect, the model was supplemented with the reversible binding of RVT to GSH into complex Y (reactions (XVII)–(XIX)).
Kinetic curves of consumption of (1–3) 1.9 mM GSH and (3) 0.53 mM RVT in the presence of 2.1 mM H2O2: (1) GSH ◆ with RVT and (2) GSH △ without RVT; aqueous medium, 37°C. The symbols denote the experimental data; the solid lines stand for the data calculated in terms of the kinetic model (Table 2).
The described kinetic model with optimized rate constants quite adequately describes the experimental concentration dependences for WRVT and WGSH (Fig. 2, Table 1) and the experimental kinetic curves of RVT and GSH consumption in the reaction between GSH and RVT in the presence of H2O2 (Figs. 1, 4).
CONCLUSIONS
Based on the experimentally obtained kinetic curves and concentration dependences of the RVT and GSH consumption rate in the presence of H2O2 on the reactant concentrations (in an aqueous medium at 37°C) and data on the product composition, a kinetic model of interaction between GSH and RVT initiated by thiyl radicals formed in the reaction between GSH and H2O2 has been developed. The skeletal model includes 19 reactions with respective rate constant values optimized for the experimental conditions. The reactions describing formation of GSH–H2O2 and GSH–GSH complexes have made it possible to describe the nontrivial concentration dependences of the GSH consumption rate and the radical initiation rate during the interaction between GSH and H2O2; reactions (XIV) and (XV) represent the important role of atmospheric oxygen in the radical chain consumption of RVT. Supplementing of the model with reversible reactions (XVII)–(XIX) describing the formation of RVT complexes with the process components has made it possible to describe the nontrivial effect of a significant decrease in the GSH consumption rate at high RVT concentrations. Most of the studies on the biochemistry of GSH are conducted under conditions close to physiological conditions in animal organisms, i.e., in buffer solutions providing a pH of 7.2–7.4. Under these conditions, no radicals are formed in the reaction between GSH and H2O2 [27]; therefore, RVT is not consumed. Apparently, the formation of radicals observed during the interaction of GSH with H2O2 and other peroxides and reactions of GSH with unsaturated phenols occur and play a role in physiology of plants in which intra- and intercellular fluids are characterized by lower pH values than those of fauna and in the use of thiols in cosmetics, pharmaceuticals, dietary supplements, and winemaking.
REFERENCES
Mironczuk-Chodakowska, I. and Witkowska, A.M., Adv. Med. Sci., 2018, vol. 63, no. 3, p. 68. https://doi.org/10.1016/j.advms.2017.05.005
Sporer, A.J., Kahl, L.J., Price-Whelan, A., and Dietrich, L.E.P., Annu. Rev. Biochem., 2017, vol. 86, p. 777. https://doi.org/10.1146/annurev-biochem-061516-044453
Hartl, J., Kiefer, P., Kaczmarczyk, A., Mittelviefhaus, M., Meyer, F., Vonderach, T., Hattendorf, B., Jenal, U., and Vorholt, J.A., Nat. Metab., 2020, vol. 2, p. 153. https://doi.org/10.1038/s42255-019-0166-0
Elgawish, M.S., Kishikawab, N., and Kurodab, N., Analyst, 2015, vol. 140, p. 8148. https://doi.org/10.1039/c5an01604e
Chen, Y., Han, M., Matsumoto, A., Wang, Y., Thompson, D.C., and Vasiliou, V., Adv. Exp. Med. Biol., 2018, vol. 1032, p. 37. https://doi.org/10.1007/978-3-319-98788-0_3
Burtis, A.C., Ashwood, E.R., Saunders, W.B., and Tietz, N.W., Fundamentals of Clinical Chemistry, Philadelphia, 1996, 4th ed.
Estrela, J.M., Ortega, A., and Obrador, E., Crit. Rev. Clin. Lab Sci., 2006, vol. 43, no. 2, p. 143. https://doi.org/10.1080/10408360500523878
Toyokuni, S., Front. Pharmacol., 2014, vol. 5, no. 200, p. 1. https://doi.org/10.3389/fphar.2014.00200
Guo, R., Yang, G., Feng, Z., Zhu, Y., Yang, P., Song, H., Wang, W., Huang, P., and Zhang, J., Biomater. Sci., 2018, vol. 6, no. 5, p. 1238. https://doi.org/10.1039/c8bm00094h
Zinatullina, K.M., Kasaikina, O.T., Kuz’min, V.A., and Khrameeva, N.P., Kinet. Catal., 2019, vol. 60, no. 3, p. 266. https://doi.org/10.1134/S0023158419030169
Zinatullina, K.M., Kasaikina, O.T. Kuzmin, V.A., Khrameeva, N.P., and Shapiro, B.I, Russ. Chem. Bull., 2017, vol. 66, no. 7, p. 1300. https://doi.org/10.1134/S0023158417050093
Zinatullina, K.M., Khrameeva, N.P., Kasaikina, O.T., and Kuzmin, V.A., Russ. Chem. Bull., 2018, vol. 67, no. 4, p. 726. https://doi.org/10.1007/s11172-018-2129-0
Zinatullina, K.M., Khrameeva, N.P., Kasaikina, O.T., Kuzmin, V.A., and Shapiro, B.I., Russ. Chem. Bull., 2017, vol. 66, no. 11, p. 2145.] https://doi.org/10.1007/s11172-017-1995-1
Zinatullina, K.M., Khrameeva, N.P., and Kasaikina, O.T., Bulg. Chem. Commun., 2018, vol. 50, p.25.
Zinatullina, K.M., Kasaikina, O.T., Kuzmin, V.A., Khrameeva, N.P., and Shapiro, B.I., Russ. Chem. Bull., 2016, vol. 65, no. 12, p. 2825. https://doi.org/10.1007/s11172-016-1663-x
Zinatullina, K.M., Kasaikina, O.T., Motyakin, M.V., Ionova, I.S., Degtyarev, E.N., and Khrameeva, N.P., Russ. Chem. Bull., 2020, vol. 69, no. 10, p. 1865. https://doi.org/10.1007/s11172-020-2971-8
Posner, T., Ber. Dtsch. Chem. Ges., 1905, vol. 38, no. 1, p. 646. https://doi.org/10.1002/cber.190503801106
Nilsson, C., Simpson, N., Malkoch, M., Johansson, M., and Malmström, E., J. Polym. Sci. A: Polym. Chem., 2008, vol. 46, no. 4, p. 1339. https://doi.org/10.1002/pola.22474
Liu, Y., Hou, W., Sun, H., Cui, C., Zhang, L., Jiang, Y., Wu, Y., Wang, Y., Li, J., Sumerlin, B.S., Liu, Q., and Tan, W., Chem. Sci., 2017, vol. 8, p. 6182. https://doi.org/10.1039/c7sc01447c
Biermann, U. and Metzger, J.O., Eur. J. Org. Chem., 2018, no. 6, p. 730. https://doi.org/10.1002/ejoc.201701692
Salehi, B., Mishra, A.P., Nigam, M., Sener, B., Kilic, M., Sharifi-Rad, M., Fokou, P.V.T., Martins, N., and Sharifi-Rad, J., Biomedicines, 2018, vol. 6, no. 3, p. 91. https://doi.org/10.3390/biomedicines6030091
Yu, W., Fu, Y.C., and Wang, W., J. Cell. Biochem., 2012, vol. 113, no. 3, p. 752. https://doi.org/10.1002/jcb.23431
Chatgilialoglu, C. and Ferreri, C., Acc. Chem. Res., 2005, vol. 38, no. 6, p. 441. https://doi.org/10.1021/ar0400847
Samadi, A., Andreu, I., Ferreri, C., Dellonte, S., and Chatgilialoglu, C., J. Am. Oil Chem. Soc., 2004, vol. 81, no. 8, p. 753. https://doi.org/10.1007/s11746-004-0974-8
Ellman, G.L., Arch. Biochem. Biophys., 1959, vol. 82, p. 70.
Pereira, C.D., Minamino, N., and Takao, T., Anal. Chem., 2015, vol. 87, p. 10785. https://doi.org/10.1021/acs.analchem.5b03431
Zinatullina, K.M., Kasaikina, O.T., Kuz’min, V.A., Khrameeva, N.P., and Pisarenko, L.M., Russ. Chem. Bull., 2019, vol. 68, p. 1441. https://doi.org/10.1007/s11172-019-2574-4
Sirick, A.V., Pliss, R.E., Rusakov, A.I., and Pliss, E.M., Oxid. Commun., 2014, vol. 37, no. 1, p. 37.
Denisov, E.T. and Denisova, T.G., Handbook of Antioxidants: Bond Dissociation Energies, Rate Constants, Activation Energies and Enthalpies of Reactions, Boca Raton: CRC, 2000, p. 289.
Deutsch, J.C., Santhosh-Kumar, C.R., and Kolhouse, J.F., J. Chromatogr. A, 1999, no. 862, p. 161. https://doi.org/10.1016/S0021-9673(99)00932-2
Winterbourn, C.C. and Metodiewa, D., Free Radicals Biol. Med., 1999, vol. 27, p. 322. https://doi.org/10.1016/S0891-5849(99)00051-9
Petzolda, H. and Sadler, P.J., Chem. Commun., 2008, p. 4413. https://doi.org/10.1039/b805358h
Singh, B., Das, R.S., Banerjee, R., and Mukhopadhyay, S., Inorg. Chim. Acta, 2014, no. 418, p. 51. https://doi.org/10.1016/j.ica.2014.03.003
Chatgilialoglu, C. and Bowry, V.W., J. Org. Chem., 2018, vol. 83, no. 16, p. 9178. https://doi.org/10.1021/acs.joc.8b01216
Abedinzadeh, Z., Gardes-Albert, M., and Ferradini, C., Can. J. Chem., 1989, vol. 67, p. 1247. https://doi.org/10.1139/v89-190
Berges, J., Caillet, J., Langlet, J., and Abedinzadeh, Z., Theor. Claim. Acta, 1993, vol. 85, p. 87 99. https://doi.org/10.1007/BF01374579
Picquart, M., Grajcar, L., Baron, M.H., and Abedinzadeh, Z., Biospectroscopy, 1999, vol. 5, p. 328. https://doi.org/10.1002/(SICI)1520-6343(1999)5:6<328::AID-BSPY2>3.0.CO;2-J
Abedinzadeh, Z., Can. J. Physiol. Pharmacol., 2001, vol. 79, p. 166. https://doi.org/10.1139/cjpp-79-1-166
Hellwege, K.-H., Madelung, O., and Martienssen, W., Landolt-Bornstein: Numerical Data and Functional Relationships in Science and Technology, Berlin: Springer, 1983, vol. 13, 308 p.
Chatgilialoglu, C. and Studer, A., Encyclopedia of Radicals in Chemistry, Biology and Materials, New York: Wiley, 2012. https://doi.org/10.1002/9781119953678
Ito, O. and Matsudo, M., J. Am. Chem. Soc., 1979, vol. 101, no. 7, p. 1815. https://doi.org/10.1021/ja00501a031
Ito, O. and Matsudo, M., J. Am. Chem. Soc., 1979, vol. 101, no. 19, p. 5732. https://doi.org/10.1021/ja00513a045
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 20-03-00753) and performed under a state task (project no. 0082-2018-0006, registration no. АААА-А18-118020890097-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by M. Timoshinina
Abbreviations: GSH, glutathione; RVT, resveratrol; Wi, thiyl radical initiation rate; DTNB, 5,5'-dithiobis(2-nitrobenzoic acid); PBS, phosphate buffered saline; WRVT, RVT consumption rate; WGSH, GSH consumption rate.
Rights and permissions
About this article
Cite this article
Zinatullina, K.M., Kasaikina, O.T., Khrameeva, N.P. et al. Interaction between Glutathione and Resveratrol in the Presence of Hydrogen Peroxide: A Kinetic Model. Kinet Catal 62, 255–263 (2021). https://doi.org/10.1134/S0023158421020130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158421020130