Abstract
Framework titanosilicates of the heterophyllosilicate family with three-layer HOH modules have a microporous structure and are characterized by the occurrence of systems of wide intersecting or parallel channels. The theoretical analysis of possible migration paths of various cations is performed using the ToposPro program. It is found that in heterophyllosilicates with astrophyllite-1A/-2M and veblenite structure types, a one-dimensional conductivity of alkali metal ions (Na+, K+, Rb+, Cs+), silver (Ag+), and lead (Pb2+) is possible through a system of wide parallel channels in the [100] direction. Lithium substitution for large alkali cations appreciably changes the character of the ionic conductivity. Due to a smaller radius, Li+ cations can pass through a window between channels, forming a two-dimensional conductive layer parallel to (001).

Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Natural and synthetic titanosilicates are of interest as promising materials having a wide spectrum of physical and chemical properties [1]. Layered, pseudo-layered, and framework titanosilicates with systems of wide channels are characterized by sorption [2] and ion-exchange properties [3-9]. The crystalline materials with framework structures containing systems of intersecting channels of different diameters usually exhibit ion-exchange properties [10]. During the ion exchange in these crystals, incoming and outgoing ions move in different channels, these processes occurring simultaneously, according to the conservation requirement of electroneutrality of the crystal. In counter flows, ion exchange is usually sterically hindered.
Framework titanosilicates of the heterophyllosilicate family [6, 11-13], whose structures are based on three-layer HOH modules, where the central O-layer is formed by edge-sharing M-octahedra and external H-layers are represented by a network of SiO4 tetrahedra and Tiφ6 octahedra (or Tiφ5 semi-octahedra), possess a microporous structure and are characterized by the occurrence of systems of wide intersecting (perrotite group) or parallel channels (astrophyllite supergroup, nafertisite group, veblenite). From the experimental data and theoretical calculations it was previously found [14] that in mineral caryochroite (nafertisite structure type), ion exchange is possible in counter flows for Na+, K+, Rb+, Cs+, Ag+, and Pb2+ ions, and for lithium ions possible exchange between the neighboring channels with the formation of two-dimensional conductivity, was also established.
The astrophyllite supergroup is formed by minerals with the general formula \({{A}_{\text{2}p}}{{B}_{r}}{{C}_{\text{7}}}{{D}_{\text{2}}}{{(\text{S}{{\text{i}}_{\text{4}}}{{\text{O}}_{\text{12}}})}_{\text{2}}}\text{I}X_{D2}^{O}X_{A4}^{O}X_{Dn}^{P}{{W}_{A}}_{\text{2}}\), where C = Fe2+, Mn, Na, Mg, Zn, Fe3+, Ca, Zr, Li are the cations located in M-sites of the octahedral О-layer; [6,5]D = Ti, Nb, Zr, Sn4+, [5]Fe3+, Mg, Al are the cations located in L-sites of the heteropolyhedral H-layer; A = K, Cs, Ba, H2O, Li, Pb2+, Na, □; p = 1, 2; B = Na, Ca, Ba, H2O, □; r = 1, 2; \(X_{D}^{O}=\text{O;}\) \(X_{A}^{O}=\text{OH}\text{,}\ \text{F;}\) \(X_{Dn}^{P}=\text{O}\text{,}\ \text{OH}\text{,}\ \text{F}\text{, }{{\text{H}}_{\text{2}}}\text{O}\text{, }\square \text{;}\) n = 0, 1, 2; WA=H2O, □; I (in the devitoite structure) = (PO4)2(CO3) [15]. This supergroup combines triclinic and monoclinic minerals [15-17] of the heterophyllosilicate family, which are characterized by a high degree of silicon polymerization expressed by the presence of wide tetrahedral ribbons Si4O12. Titanium atoms are in the octahedral coordination (being involved in the combination of neighboring HOH modules into a heteropolyhedral quasi-framework through common oxygen vertices) and in the semi-octahedral (tetragonal-pyramidal) coordination. Using the structure generation function SN.n [13], the general formula of the astrophyllite HOH module can be written as
(HOH)(Astro) = {[6]M7⊘4[[5-6]LO(Si4O12)Θn]2},
where M are cations of the octahedral O-layer; ⊘-ligands (\(X_{A}^{O}\)-anions) belong to the octahedral O-layer, and Θ-ligands (\(X_{D}^{P}\)-anions) are apical (n = 1) or bridging (n = 0.5) vertices of Lφ6 octahedra of the neighboring HOH modules (in the case of LO5 semi-octahedra, n = 0). A negative charge of the astrophyllite HOH module is compensated by alkali and alkaline earth А-cations located, along with water molecules, either in the interlayer space (in the case of isolated HOH modules) or wide channels stretched in the [100] direction (in the case of the combination of neighboring HOH modules).
With regard to different coordinations of titanium, and also possible ways of combining HOH modules (straight through the vertices of Tiφ6 octahedra), four structure types may be distinguished for minerals of the astrophyllite group [15-19].
1. The astrophyllite structure type in which HOH modules are combined via bridging Θ-ligands of Lφ6 octahedra (n = 0.5). This structure type can have two polytypes that differ in combination features of the neighboring HOH modules, and consequently, symmetry and unit cell parameters [20]:
а) triclinic 1А-polytype (a ~ 5.4 Å, b ~ 11.9 Å, c ~ 11.7 Å, α ~ 113.0°, β ~ 94.5°, γ ~ 103.1°; space group \(P\bar{1}\)) (Fig. 1а);
b) monoclinic 2M-polytype (a ~ 5.4 Å, b ~ 23.2 Å, c ~ 21.2 Å, β ~ 95.2°; space group C2/c) (Fig. 1b).
2. Lobanovite sructure type in which Θ-ligands are absent (n = 0), and the neighboring HOH modules are combined by A-cations (a ~ 5.3 Å, b ~ 23.2 Å, c ~ 10.4 Å, β ~ 96.6°; space group C2/m) (Fig. 1с).
3. Sveinbergeite structure type in which Θ-ligands are present (n = 1), but the neighboring HOH modules are combined by A-cations (a ~ 5.3 Å, b ~ 11.8 Å, c ~ 11.8 Å, α ~ 101.1°, β ~ 98.2°, γ ~ 102.4°; space group \(P\bar{1}\)) (Fig. 1d).
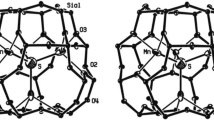
Veblenite \({{\text{K}}_{\text{2}}}\text{Na}(\text{Fe}_{5}^{2+}\text{Fe}_{4}^{3+}\text{Mn}_{7}^{2+}\square )\text{N}{{\text{b}}_{\text{3}}}\text{Ti}{{(\text{S}{{\text{i}}_{\text{2}}}{{\text{O}}_{\text{7}}})}_{\text{2}}}{{(\text{S}{{\text{i}}_{\text{8}}}{{\text{O}}_{\text{22}}})}_{\text{2}}}{{\text{O}}_{\text{6}}}{{(\text{OH})}_{\text{1}0}}{{({{\text{H}}_{\text{2}}}\text{O})}_{\text{3}}}\) [21] is complex titanosilicate of the heterophillosilicate family whose HOH module is based on wide silicon-oxygen ribbons of the типа Si8O22 veblenite type as well as Si2O7 diorthogroups (Fig. 2). Using SN.n, we can write the general formula of the HOH module as follows [13, 21]:
(HOH)(Veb) = {[6]M17⊘10[[6]L2O2(Si2O7)(Si8O22)Θ*]2}.
Owing to the presence of veblenite chains in the H-layer of the HOH module, the veblenite structure contains even wider channels than those in the nafertisite structure type [14].
General view of the crystal structure of astrophyllite group minerals: astrophyllite-1А (а), astrophyllite-2М (b), lobanovite (c), and sveinbergeite (d) (in the figure: MO6 octahedra of the O-layer are shown by gray; SiO4 tetrahedra of the H-layer by yellow; light blue circles designate sodium; green are potassium; dark gray are calcium; large dark blue circles designate water molecules) (see el. version).
It was shown previously that under supercritical conditions (in the temperature range of 400-600 °C and a pressure of 1000 kg/cm2), astrophyllite exhibited ion exchange properties. In particular, under these conditions Na+, Rb+, and Cs+ ions can substitute for K+ ions [22, 23]. Under milder natural conditions, astrophyllite can be involved in ion exchange reactions with aqueous solutions, as a result of which, oxonium ions and water molecules substitute for Na+ ions with the formation of so-called hydroastrophyllite (H3O,H2O,K,Ca)3(Fe,Mn)5-6Ti2Si8(O,OH)31 [24], however, these processes take place over a long geological time. The ion exchange properties of lobanovite, sveinbergeite, and veblenite have not been studied experimentally. Nonetheless, it is possible to assume that due to their crystal structures, ion exchange is unlimited because by analogy with micas and other clay minerals with layered structures [11, 25], the interlayer space can expand depending on the cation type (e.g., in devitoite, CO3 and PO4 groups are located in the interlayer space [26]).
The aim of this work was the theoretical analysis of possible migration paths of various cations (Na+, K+, Rb+, Cs+, Ag+, Pb2+, as the most common) in wide channels of framework representatives of the astrophyllite supergroup, as well as veblenite.
THEORETICAL CALCULATIONS
To analyze ion migration paths in astrophyllite group minerals using the ToposPro program package [27] we employed structure models of astrophyllite-1A [18], astrophyllite-2M [18], and veblenite [21]. This approach is based on the Voronoi–Dirichlet partition that makes it possible to obtain an adequate map of the system of cavities and channels [28]. The result of this partition is two interpenetrating graphs: atomic and void networks. The elementary void radius (Rsd) is calculated as a radius of the sphere whose volume equals the void volume of the Voronoi–Dirichlet polyhedron (VDP). By comparing the elementary void radius (Rsd) with the radius of a mobile atom in the respective environment [29, 30], it is possible to determine the probability of the atom location in this void. The elementary channel radius (Rchan) is calculated as the arithmetic mean of distances between the center of gravity of the elementary channel cross-section and atoms forming it. In estimating the channel radius, it is needed to multiply the sum of radii of the mobile cation and the atom forming the channel (oxygen) by the deformation coefficient γ [31] taking into account the possible polarization of ions during their passing through the channel [32-34].
Since not all voids and channels are significant (i.e., available for the migration of cations of a certain type), it is necessary to sort them out using the significance parameters found from VDP calculations of the known cation conductors. Table 1 lists the significance parameters for the void and channel radii if the migrating cation is Li+, Na+, K+, Rb+, Cs+, Ag+, or Pb2+. Voids and channels having the radius smaller than the respective criteria together with channel systems were removed. A set of significant elementary voids and channels linking them forms the migration map describing all possible diffusion paths of the mobile cation [29, 30].
RESULTS AND DISCUSSION
Astrophyllite supergroup minerals with astrophyllite-1A and astrophyllite-2M structure types contain a system of wide parallel channels whose effective width (calculated as O⋯O distances determining the channel diameter minus 2.7 Å [35]) is 1.48×9.22 Å and is characterized by the 12-member cross-section (Fig. 3). According to recommendations of the International Zeolite Association and IUPAC, the crystal chemical formula of ordered microporous compounds can be represented in the form [35, 36]: |guest composition| [host composition] h{host dimensionality} p{characteristic of pores/channels} (symmetry, space group). Thus, their crystal chemical formula should be written as (Z = 2)
|A2nBm(H2O))k| [[6]M7⊘4[[6]LO(Si4O12)Θn]2] h{3} g{1[66122/2][100]} (\(P\bar{1}\) or C2/c).
This formula reflects that the structures are based on HOH modules of the astrophyllite type, which combine and form a system of parallel channels directed along the x axis; guests of the structure are large A- and B-cations characterized by different charges and coordination numbers, as well as water molecules; n = 1, 2; m = 1, 2; k = 0-2.
By the topological analysis it is found that framework representatives of the astrophyllite supergroup, which correspond to astrophyllite-1A and astrophyllite-2M structure types, have one-dimensional conductivity channels of Na+, K+, Ag+, Pb2+, and Rb+ ions along the [100] direction (Fig. 4а), whereas the Cs+ ion is too large and cannot migrate along the channel. The centers of six-member rings of H-layers contain large voids linked with the central systems of channels suitable for the accommodation of large cations in the case of ion exchange in counter flows. As established previously for the nafertisite structure type [14], despite that a six-member window with the B-position in its center, which is occupied mainly by sodium, is present between the pairs of Tiφ6 octahedra, the cation exchange between the neighboring channels does not occur through it because of a number of steric hindrances related, first of all, to its effective sizes.
The ion conductivity changes from one-dimensional to two-dimensional when Li+ ions are used: owing to the small radius, they can migrate between the neighboring channels through the B-position in astrophyllite-1А and astrophyllite 2М structure types (Fig. 4b), similarly to the nafertisite structure type [14]. As in the case of large cations, the six-member rings of H-layers contain voids suitable for Li+ ions linked with the central conductive layer and can be occupied during the ion exchange in countercurrent flows.
The veblenite crystal structure has two types of channels: narrow and wide with cross-sections having effective sizes of 0.9×5.0 Å (hexagonal) and 4.1×17.2 Å (icosagonal), respectively (Fig. 5). The crystal chemical formula of veblenite can be written as (Z = 2)
|A3B2(H2O)k| [M17⊘10[[6]L2O2(Si2O7)(Si8O22)Θ*]2] h{3} p{1[610202/2][100]/1[64/2][100]}\((P\bar{1})\),
where A = K, □; B = Na, □.
Due to the presence of tetrahedral ribbons of the veblenite type in the Н-layer, the veblenite crystal structure contains the widest channels known at present among the representatives of the heterophyllosilicate family. In the narrow channel, the migration of ions with a sufficiently small radius, e.g. Na+, is also possible. With an increase in the ion radius its width becomes insufficient, and already for the Ag+ ion there are only separate voids within the channel that can accommodate it. For Pb2+ and cations with a larger ionoc radius the narrow channel is completely excluded from the conductive system. Effective sizes of the wide channel in the veblenite structure provide the ion exchange for all ions in counter flows, including large Cs+. For Li+ ions the conductivity becomes two-dimensional due to the possible migration of ions through the respective windows between the neighboring channels (similarly to the astrophyllite and nafertisite structure types).
A comparative characteristic of the migration paths for different cations in titanosilicates of the heterophyllosilicate family is presented in Table 2.
CONCLUSIONS
By topological calculations it is found that in heterophyllosilicates with astrophyllite-1A/-2M and veblenite structure types, the one-dimensional character of the ion conductivity of alkali metals (Na+, K+, Rb+, Cs+), silver (Ag+), and lead (Pb2+) can occur through the system of wide parallel channels in the [100] direction. The neighboring channels are separated by walls of connected Tiφ6 octahedra from H-layers, which hinder the cation exchange between the channels. Lithium substitution for large alkali cations substantially changes the character of the cation conductivity. Owing to the smaller radius, Li+ ions can pass through the window between the channels, forming a two-dimensional conductive layer parallel to (001). For purely layered structures similar to micas and clay materials it may be assumed that the ion exchange as well as the sorption capacity are unlimited because the interlayer space can expand depending on the ion type and/or the respective cation or anion group, and also a neutral molecule.
Change history
16 June 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S0022476622040205
REFERENCES
N. V. Chukanov, I. V. Pekov, and R. K. Rastsvetaeva. Russ. Chem. Rev., 2004, 73, 205. https://doi.org/10.1070/RC2004v073n03ABEH000825
K. Popa and C. C. Pavel. Desalination, 2012, 293, 78. https://doi.org/10.1016/j.desal.2012.02.027
J. Rocha and Z. Lin. Rev. Mineral. Geochem., 2005, 57, 173. https://doi.org/10.2138/rmg.2005.57.6
J. Rocha and M. W. Anderson. Eur. J. Inorg. Chem., 2000, 2000, 801. https://doi.org/10.1002/(SICI)1099-0682(200005)2000:5%3C801::AID-EJIC801%3E3.0.CO;2-E
S. M. Kuznicki, V. A. Bell, S. Nair, H. W. Hillhouse, R. M. Jacubinas, C. M. Braunbarth, B. H. Toby, and M. Tsapatsis. Nature, 2001, 412, 720. https://doi.org/10.1038/35089052
Z. Lin, F. A. A. Paz, and J. Rocha. In: Layered Mineral Structures and their Application in Advanced Technologies / Eds. M. F. Brigatti and A. Mottana. Mineralogical Society of Great Britain and Ireland, 2011, 123. https://doi.org/10.1180/EMU-notes.11.3
G. Ferraris, A. Bloise, and M. Cadoni. Microporous Mesoporous Mater., 2008, 107, 108. https://doi.org/10.1016/j.micromeso.2007.02.036
I. S. Lykova, N. V. Chukanov, A. I. Kazakov, V. P. Tarasov, I. V. Pekov, V. O. Yapaskurt, and N. A. Chervonnaya. Phys. Chem. Mineral., 2013, 40, 625. https://doi.org/10.1007/s00269-013-0598-0
I. S. Lykova, I. V. Pekov, N. V. Zubkova, N. V. Chukanov, V. O. Yapaskurt, N. A. Chervonnaya, and A. A. Zolotarev. Eur. J. Mineral., 2015, 27, 535. https://doi.org/10.1127/ejm/2015/0027-2445
A. Zagorodni. Ion Exchange Materials: Properties and Applications. Elsevier, 2006. https://doi.org/10.1016/B978-008044552-6/50006-X
G. Ferraris and A. Gula. Rev. Mineral. Geochem., 2005, 57, 69. https://doi.org/10.2138/rmg.2005.57.3
R. K. Rastsvetaeva and S. M. Aksenov. Crystallogr. Rep., 2011, 56. https://doi.org/10.1134/S1063774511060216
F. C. Hawthorne. Mineral. Mag., 2012, 76, 1053. https://doi.org/10.1180/minmag.2012.076.5.13
N. V. Chukanov, S. M. Aksenov, I. V. Pekov, N. A.Chervonnaya, D. A.Varlamova, V. N. Ermolaeva, and S. N. Britvin. Microporous Mesoporous Mater., 2021, 312, 110776. https://doi.org/10.1016/j.micromeso.2020.110776
E. Sokolova, F. Cámara, F. C. Hawthorne, and M. E. Ciriotti. Mineral. Mag., 2017, 81, 143. https://doi.org/10.1180/minmag.2016.080.077
P. C. Piilonen, A.r.E. Lalonde, A. M. McDonald, R. A. Gault, and A. O. Larsen. Can. Mineral., 2003, 41, 1. https://doi.org/10.2113/gscanmin.41.1.1
N. A. Yamnova and S. M. Aksenov. Crystallogr. Rep., 2021, 66(7), 1167. https:// doi.org/10.1134/S106377452107021X
P. C. Piilonen, A. M. McDonald, and A. E. Lalonde. Can. Mineral., 2003, 41, 27. https://doi.org/10.2113/gscanmin.41.1.27
E. Sokolova. Mineral. Mag., 2012, 76, 863. https://doi.org/10.1180/minmag.2012.076.4.04
P. C. Piilonen, A. M. McDonald, and A. E. Lalonde. Eur. J. Mineral., 2001, 13, 973. https://doi.org/10.1127/0935-1221/2001/0013/0973
F. Cámara, E. Sokolova, F. C. Hawthorne, R. Rowe, J. D. Grice, and K. T. Tait. Mineral. Mag., 2013, 77, 2955. https://doi.org/10.1180/minmag.2013.077.7.06
N. F. Chelishchev. Geokhimiya, 1972, 7, 856.
N. F. Chelishchev. Ionoobmennye svoistva mineralov (Ion Exchange Properties of Minerals). Nauka, 1973. [In Russian]
X-ray Laboratory. Hubei (Hupei) Geologic College. Sci. Geol. Sin., 1974, 1, 18.
P. Cool, E. F. Vansant, G. Poncelet, and R. A. Schoonheydt. In: Handbook of Porous Solids / Eds. F. Schüth, K. S. W. Sing, and J. Weitkamp. Wiley-VCH Verlag GmbH, 2002, 1250.
A. R. Kampf, G. R. Rossman, and I. M. Steele. Can. Mineral., 2010, 48, 29. https://doi.org/10.3749/canmin.48.1.29
V. A. Blatov, A. P. Shevchenko, and D. M. Proserpio. Cryst. Growth Des., 2014, 14, 3576. https://doi.org/10.1021/cg500498k
N. A. Anurova and V. A. Blatov. Acta Crystallogr., Sect. B: Struct. Sci., 2009, 65, 426. https://doi.org/10.1107/S0108768109019880
V. A. Blatov, G. D. Ilyushin, O. A. Blatova, N. A. Anurova, A. K. Ivanov-Schits, and L. N. Demyanets. Acta Crystallogr., Sect. B: Struct. Sci., 2006, 62, 1010. https://doi.org/10.1107/S0108768106039425
V. A. Blatov and A. P. Shevchenko. Acta Crystallogr., Sect. A: Found. Crystallogr., 2003, 59, 34.
V. I. Voronin, M. G. Surkova, G. S. Shekhtman, N. A. Anurova, and V. A. Blatov. Inorg Mater., 2010, 46, 1234-1241. https://doi.org/10.1134/S0020168510110130.
N. A. Anurova and V. A. Blatov. Neorg. Mater., 2010, 46, 1360. [In Russian]
S. S. Fedotov, N. A. Kabanova, A. A. Kabanov, V. A. Blatov, N. R. Khasanova, and E. V. Antipov. Solid State Ionics, 2018, 314, 129-140. https://doi.org/10.1016/j.ssi.2017.11.008
R. A. Eremin, N. A. Kabanova, Y. A. Morkhova, A. A. Golov, and V. A. Blatov. Solid State Ionics, 2018, 326, 188. https://doi.org/10.1016/j.ssi.2018.10.009
L. B. McCusker, F. Liebau, and G. Engelhardt. Microporous Mesoporous Mater., 2003, 58, 3. https://doi.org/10.1016/S1387-1811(02)00545-0
F. Liebau. Microporous Mesoporous Mater., 2003, 58, 15. https://doi.org/10.1016/S1387-1811(02)00546-2
Funding
The work was supported by the Russian Science Foundation, grant No. 20-77-10065 (theoretical topological analysis of migration paths).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 2, pp. 224-232.https://doi.org/10.26902/JSC_id88422
The original online version of this article was revised: Modification has been made to the Graphical Abstract. Full information regarding the corrections made can be found in the erratum for this article.
Rights and permissions
About this article
Cite this article
Aksenov, S.M., Yamnova, N.A., Chukanov, N.V. et al. THEORETICAL ANALYSIS OF CATION- MIGRATION PATHS IN MICROPOROUS HETEROPHYLLOSILICATES WITH ASTROPHYLLITE AND VEBLENITE TYPE STRUCTURES. J Struct Chem 63, 293–301 (2022). https://doi.org/10.1134/S002247662202010X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002247662202010X