On crystals containing growth defects, the “burst-like growth” mode, i.e., a sharp acceleration of the growth of facets, has been implemented. This has confirmed the hypothesis that the same physical mechanisms are responsible for the transition of facets in the state of anomalously rapid growth at high and low temperatures. It has been found that the transition from the fast to slow kinetics of crystal facet growth is stepwise. It has been established that the relaxation of the kinetic coefficient of growth is similar to the relaxation of the elastic moduli of the crystal after the fast growth stage. The kinetic coefficients of crystal growth at fast and slow growth stages have been determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
A striking growth of helium crystals was discovered in 1996: the growth of the facets of a 4He single crystal was surprisingly accelerated above the threshold supersaturation pb depending on the temperature. In a series of experiments in the temperature range of 2–250 mK [1, 2], the c facet of a crystal without growth defects was at rest before this supersaturation was reached. Then, rapid growth occurred accompanied by a sharp drop of the pressure. The displacement of the c facet was detected by an optical method. In a series of experiments in the temperature range of 0.4–0.75 K, the crystal was nucleated in a metastable superfluid liquid [3]. The kinetics of its growth in this temperature range was determined by the most slowly growing segments of the surface, i.e., by the c and a facets. The crystal grew in the form of a hexagonal prism. After nucleation, the crystal grew slowly and the pressure in the container decreased gradually. Suddenly, the kinetic coefficient of growth for all facets increased stepwise by two or three orders of magnitude. As a result, the rapid growth of the entire crystal occurred in a time of about 200 µs, which was accompanied by the abrupt drop of the pressure. The shape and size of the crystal at this stage were detected by video recording. The review of experimental methods, phase diagram \(\Delta {{p}_{{\text{b}}}}(T)\) demonstrating the region of appearance of the anomalously rapid growth of crystals, plots of the probability of appearance of the burst-like growth mode and return to the normal state, the effect of impurities, etc., can be found in [4].
The conditions of appearance of anomalous growth have already been well studied. According to the video recording of the shape of crystals at the rapid growth stage, the transition of the a and c facets to the rapid kinetics occurs with a time delay less than 40 µs, see [4, Sect. 4.4]. The situation with the inverse process is less definite. According to the pressures measured by a pressure gauge with a low time resolution in the experiments in [1, 2], a facet returns to the initial immobile state after about 1 s. In the experiments reported in [4, Sect. 4.5], the time range was narrowed and it was found that return to the normal slow growth kinetics occurs after 20–100 ms.
The similarity of phase diagrams, the probabilistic character of the appearance of the burst-like growth mode, and the effect of small impurity on the phase diagram \(\Delta {{p}_{{\text{b}}}}(T)\) allow assuming that the same mechanism is responsible for the phenomenon in different temperature ranges. Nevertheless, this problem remains open. A growth mode similar to that observed in [1, 2] has not yet been experimentally reproduced in the temperature range of 0.4–0.75 K. Thus, only the single transition from the normal growth mode to the anomalous one was observed in those experiments. Attempts to create a high supersaturation in the container with the crystal in the normal state were unsuccessful for the following reason. Unlike crystals in [1, 2], crystals nucleated in a metastable liquid contained growth defects [5]. For this reason, when the liquid was introduced into the container, the crystal grew, absorbing most of the introduced liquid and the necessary supersaturation was not reached.
Measurements reported in this work were performed using a method where restrictions of preceding experiments are partially overcome. The internal volume of the container was reduced by a factor of about 50; as a result, the size of the crystal and, correspondingly, the area of its growth surface decreased. The impedance of the inlet capillary was reduced by an order of magnitude. This allowed significantly increasing the helium flow to the container at the same parameters of the external pressure generation system.
2 EXPERIMENTAL METHOD AND RESULTS
Crystals were grown in the container using the method developed in [4, Sect. 2]. The internal volume of the container was 80 mm3. A tungsten needle for the nucleation of crystals in the metastable liquid was placed in the center of the container. One of the walls of the container was the membrane of a capacitive sensor with a response time of 160 µs. The fundamental difference of this method from the preceding one is that the nucleation of the crystal and its following growth occur as the liquid is continuously introduced into the container. Experiments were performed at temperatures of 0.49 and 0.74 K. The upper limit of supersaturation is determined by the spontaneous nucleation of the crystal on the internal wall of the container. In these experiments, the supersaturations are in the range of 0.1–5 mbar. The kinetic coefficient of growth K is determined from the expression
where \({{V}_{{{\text{surf}}}}}\) is the surface growth rate; ρ and \(\rho {\kern 1pt} '\) are the densities of the liquid and solid helium, respectively; and \(\Delta \rho = \rho {\kern 1pt} '\; - \rho > 0\). The supersaturation \(\Delta p\) is measured from the pressure of phase equilibrium. The liquid flow into the container results in the continuous growth of the crystal, \({{V}_{{{\text{surf}}}}} \approx {\text{const}}\). The created supersaturation is inversely proportional to the kinetic coefficient of growth K. Thus, the normal slow growth of the crystal occurs at high supersaturations, whereas the supersaturation in the burst-like growth mode is orders of magnitude lower. Both these processes are seen in the time dependence of the pressure.
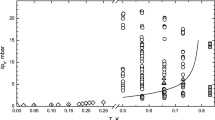
The supersaturation \(\Delta {{p}_{0}}\) at the nucleation of the crystal determines its growth mode. Figure 1 shows the kinetic coefficients of growth for a series of 24 crystals at 0.74 K. As seen in Fig. 1, 12 crystals demonstrate normal growth, and 12 crystals show an anomalously rapid growth. The kinetic coefficient of growth for crystals whose growth begins with supersaturation less than about 2.5 mbar is K = (0.28 ± 0.16) s/m. At initial supersaturations above 3 mbar, the average kinetic coefficient of growth is (2.07 ± 0.20) s/m. The threshold supersaturation \(\Delta {{p}_{{\text{b}}}}\) is in the range of 2.5–3.5 mbar, which is half the previously observed threshold supersaturation (see Fig. 11 in [4]). At a temperature of 0.49 K, only the upper bound was determined as \(\Delta {{p}_{{\text{b}}}} < 1\) mbar, which is also at least half the previously obtained value.
Average kinetic coefficient of growth versus the initial supersaturation \(\Delta {{p}_{0}}\) at T = 0.74 K. Empty circles correspond to crystals with anomalously rapid growth. Filled circles correspond to crystals with the normal slow kinetics of facet growth caused by growth defects. The half-filled circle is the kinetic coefficient of growth calculated from the envelope \(\Delta p(t)\) shown by the lower line in Fig. 2a.
Figure 2 shows the time dependence of the pressure in the container during the crystal growth at two temperatures. A critical nucleus appears at the time t = 0. The crystal rapidly grows and the pressure drops sharply to the pressure of phase equilibrium. At a temperature of 0.74 K, the supersaturation decreases monotonically. The kinetic coefficient of growth presented in the preceding paragraph was calculated from the dependence \(\Delta p(t)\). At T = 0.49 K, the kinetics of facet growth increases so that it leads to the oscillatory growth of the crystal (see [4, Sect. 2.5]), which relaxes in ~10 ms. The estimate from the ratio of the amplitude of the first oscillation period to the initial pressure at this temperature is K = 6–7 s/m. The kinetic coefficients of growth and their temperature dependence are in agreement with the results obtained in [4, Sect. 3].
Time dependence of the pressure in the container during the crystal growth at the temperature T = (a) 0.74 and (b) 0.49 K. The line with the jump at t = 0 is the pressure change at the growth of a particular crystal. The smooth line shifted downward by the arrow is the result of averaging over a series of measurements with the same initial conditions. The inset shows stepwise pressure changes similar to the burst-like growth mode on a magnified scale [1, 2].
As seen in Fig. 2, short jumps are imposed on the smooth variation of the pressure. The slow component is the same for crystals grown under the same initial conditions. The lower lines in Fig. 2 are averaged dependences \(\Delta p(t)\) over a series of experiments under the same initial conditions matched in the time of beginning of growth and normalized. Such processing averages the quasiperiodic jumps of the pressure. It is seen that the supersaturation at both temperatures increases, reaches a maximum at about 30 ms, and then after about 250 ms approaches zero. Figure 3 shows the dependences of the kinetic coefficient of growth K calculated using the method proposed in [6]. First, the kinetics relaxes to the stationary state. These segments of \(K(t)\) are well approximated by an exponential dependence with a time constant of 5 and 7 ms at a temperature of 0.74 and 0.49 K, respectively. The kinetic coefficient of growth becomes almost constant after about 30 ms. The kinetic coefficient of growth at a temperature of 0.74 K is K = (0.030 ± 0.006)) s/m (see the half-filled circle in Fig. 1). Cooling to 0.49 K increases the kinetic coefficient of growth by a factor of more than 2 to the value K = (0.07 ± 0.007)) s/m.
Abrupt drops of the pressure observed in the interval of 30–250 ms indicate a rapid growth of the crystal, i.e., the transition of the facets of the crystal to an anomalous state. According to the dependences \(\Delta p(t)\) in Fig. 2, jumps at a temperature of 0.74 K appear at supersaturations in the range of 0.3–0.8 mbar. As the temperature is reduced, this range is shifted downward to 0.05–0.2 mbar. The temperature affects the frequency of appearance of jumps, as seen in the inset of Fig. 2. Jumps at \(T = 0.49\) K appear more frequently than at \(T = 0.74\) K.
The monotonic increase in the supersaturation after a jump is due to the return of the facets of the crystal to the normal state with slow kinetics. The dependences \(\Delta p(t)\) show that return to the normal state occurs without a significant delay, which does not exceed the response time of the pressure measurement system of 160 µs. The shape of the pressure jumps is asymmetric. After the appearance of the burst-like growth mode at \(T = 0.74\) K, the pressure drops in about 400 µs. This time is determined by the kinetic coefficient of growth and corresponds to the rapid growth after the nucleation of the crystal. Supersaturation returns to the initial value in 4–5 ms. The pressure drop time at a temperature of 0.49 K is 200–250 µs. It is noteworthy that this time is determined in this case by the frequency of soft oscillation modes of the pressure at the oscillatory growth of the crystal rather than by the kinetic coefficient of growth [4, Sect. 2.5]. The supersaturation recovery time decreases to 0.6–0.8 ms. After about 250 ms, the supersaturation decreases and the burst-like growth mode ends.
3 DISCUSSION OF THE RESULTS
The experiments showed that the burst-like growth mode, which was previously observed for perfect crystals, is also implemented for crystals with growth defects. The anomalously rapid crystal growth mode is manifested identically in both higher, 0.4–0.75 K, and lower, 2–250 mK, temperature ranges. This is a strong reason for the physical identity of both effects. Although this conclusion does not clarify the physical mechanism responsible for the effect, it allows eliminating many possible explanations. For example, since the effect is observed for a perfect crystal facet [1, 2], all the possible explanations associated with the presence of growth defects should be removed.
The only difference of this experiment from previous ones is that the container volume is about two orders of magnitude smaller. Since the volume of the crystal is proportional to the internal volume of the container, the final sizes of the crystals in these experiments are about one fifth of the sizes of previously studied crystals. These results are insufficient to state that just the sizes of the crystal provide this effect on the boundary \(\Delta {{p}_{{\text{b}}}}(t)\) separating the normal and anomalous regions. This is only an assumption.
The kinetic coefficients of growth at the rapid growth stage calculated from the pressure drop after the crystal nucleation are in agreement with the previously measured values. Thus, the rapid growth is identical for conditionally “large” and “small” crystals.
The drop of the kinetic coefficient of growth in about 30 ms after the end of the rapid growth stage demonstrates the relaxation of the burst-like growth mode to the normal slow growth mode. It is noteworthy that the real and imaginary parts of the elastic modulus of the crystal relax during the same time (see [4, Sect. 4.7.2]). The relaxation time of this process is 3–4 ms and varies slightly in the temperature range of 0.4–0.75 K. The existing experimental data are insufficient to answer whether this coincidence is accidental or these are two aspects of a single process.
As seen in Fig. 1, the kinetic coefficients of growth for the crystals grown in the normal state are an order of magnitude smaller than the coefficients K at the rapid growth stage. The kinetic coefficients of growth at \(T \approx 0.75\) K measured by different authors are in a wide range from 4 × 10–4 to 0.02 s/m (see Fig. 4 in [4]). This spread is not surprising because the facets of the crystal grow in this regime owing to growth defects, and the defect structure strongly depends on the mechanisms of crystal growth, annealing, etc., which are individual for each experiment. The effect of the concentration of growth defects on kinetics is clearly manifested in the different growth rates of equivalent a facets in the process of free crystal growth [5]. High growth rates of small crystals are possibly due to their defect structure. It is noteworthy that the stationary kinetic coefficients of growth calculated from the pressure envelope are an order of magnitude lower than those for the normal growth of crystals in these experiments (see Fig. 3). They are close to the values previously obtained in the works of other authors.
4 CONCLUSIONS
To summarize, the burst-like growth mode previously observed in [1, 2] has been reproduced on crystals containing growth defects. This indicates that the same physical mechanisms are responsible for the transition of facets in the state of anomalously rapid growth in the temperature range from 2 mK to 0.75 K. It has been found that the transition from the fast to slow kinetics is stepwise or, at least, occurs in a time less than about 200 µs. It has been established that the relaxation of the kinetic coefficient of growth is similar to the relaxation of the elastic moduli of the crystal after the fast growth stage. The kinetic coefficients of crystal growth at fast and slow growth stages have been determined.
REFERENCES
P. J. Hakonen, J. S. Penttila, J. P. Ruutu, J. P. Saramaki, G. Tvalashvili, A. V. Babkin, and A. Ya. Parshin, Phys. Rev. Lett. 76, 4187 (1996).
P. J. Hakonen, J. P. Ruutu, G. Tvalashvili, A. V. Babkin, and A. Ya. Parshin, J. Low Temp. Phys. 112, 117 (1998).
V. L. Tsymbalenko, Phys. Lett. A 211, 177 (1996).
V. L. Tsymbalenko, Phys. Usp. 58, 1059 (2015).
V. L. Tsymbalenko, J. Low Temp. Phys. 21, 120 (1995).
V. L. Tsymbalenko, J. Exp. Theor. Phys. 99, 1214 (2004).
ACKNOWLEDGMENTS
I am grateful to V.V. Dmitriev for the possibility of performing this work at the Kapitza Institute for Physical Problems, to V.V. Zav’yalov for support of this work, to S.N. Burmistrov for stimulating discussions, and to V.S. Kruglov for interest in the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by R. Tyapaev
Rights and permissions
About this article
Cite this article
Tsymbalenko, V.L. Observation of the “Burst-Like Growth” Mode on 4He Crystals Nucleated in a Metastable Liquid. Jetp Lett. 113, 30–33 (2021). https://doi.org/10.1134/S0021364021010100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0021364021010100