Abstract—
Aluminum-based metallic ribbons with the compositions Al86Ni4Co4Nd(Sm)6 and Al86Ni6Co2Nd(Sm)6 were produced by melt spinning. X-ray diffraction characterization showed that the ribbons had an amorphous structure. Their crystallization kinetics were studied by differential scanning calorimetry and their resistivity was measured by a standard four probe method. The Al–Ni–Co–REM ribbons were shown to have a broader temperature range of an amorphous state than do Al–Ni(Co)–REM ternary alloys. We found compositions with an enhanced glass-forming ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Aluminum-based amorphous ribbons containing transition metals (TM = Ni or Co) and rare-earth metals (REMs) offer a unique combination of mechanical properties and corrosion resistance [1–5]. If nickel is used as a transition metal, the amorphous alloys possess improved mechanical properties (their tensile strength reaching 1000 MPa) [1]. At the same time, cobalt-containing ribbons demonstrate high corrosion resistance [6]. It is reasonable to expect that combining these elements in amorphous Al–Ni–Co–REM alloys will ensure the formation of metallic glasses with improved mechanical properties and corrosion resistance.
The purpose of this work is to study how the presence of Nd and Sm and the content of 3d transition metals influence glass formation in Al–Ni–Co–REM alloys (containing 4–6 at % Ni, 2–4 at % Co, and 6 at % Nd or Sm) and their electrical properties.
EXPERIMENTAL
Alloys for amorphous ribbons were prepared by melting starting materials in an induction furnace at 1923 K over a period of 0.5 h under an argon atmosphere. The chemical composition of the alloys was determined on an atomic absorption spectrometer. Amorphous ribbons (2 mm in width and 36–45 μm in thickness) with the compositions Al86Ni4Co4Nd6, Al86Ni6Co2Nd6, Al86Ni4Co4Sm6, and Al86Ni6Co2Sm6 were produced by melt spinning under a controlled inert gas atmosphere. First, we evacuated air from the chamber, and then it was filled with argon to a pressure of 103 Pa. The melt was superheated to 1500–1523 K in the induction furnace and then jetted onto a water-cooled copper drum.
The structure of the resultant samples was studied by X-ray diffraction (CuKα radiation) on a Bruker D8 Advance diffractometer. They were characterized by differential scanning calorimetry (DSC) at heating rates of 10, 20, and 40 K/min using a PerkinElmer DSC-7 system. The resistivity of the ribbons was measured by the four probe method at a heating rate of 10 K/min. The experimental procedure was described in detail elsewhere [7].
RESULTS AND DISCUSSION
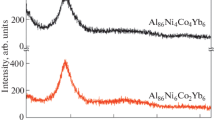
According to X-ray diffraction data, the ribbons had an amorphous structure (Fig. 1): no Bragg peaks were detected in their X-ray diffraction patterns. The main peak of the Al86Ni4Co4Nd6 and Al86Ni6Co2Nd6 ribbons is located at 2θ = 38°–39°, that is, at a 10° smaller angle in comparison with a ribbon containing 8 at % Ni [8]. The prepeak located to the left of the main peak and characteristic of nickel-containing ribbons [8] is shifted by a small angle. No such peak was detected in our experiments. The presence of Sm instead of Nd has no effect on the position of the main peak, but its intensity is higher by 50 units in the case of the neodymium-containing ribbons.
Figures 2 and 3 show typical DSC curves obtained at different heating rates.
All of the alloys were shown to have three exothermic peaks. In addition, the Al86Ni6Co2Sm6 alloy was found to have a fourth exothermic peak, with a small heat effect. The presence of Sm instead of Nd, at different relative amounts of the 3d transition metals, reduces not only the temperature of the first stage of crystallization (by 15–20 K) bur also the corresponding heat effect.
Table 1 lists the temperatures of the observed peaks in the DSC curves of the alloys.
Increasing the nickel content of the neodymium-containing ribbons to 6 at % was shown to reduce the temperatures of all three stages of crystallization. The temperatures of the first and second peaks were lower by 30 K, and that of the third peak decreased by 23 K. In addition, the heat effect of the first stage was smaller than that in the case of the alloy containing 4% Ni, whereas the heat effects of the second and third stages were larger. The third peak of these ribbons had a simple shape. Moreover, the glass transition in the Al86Ni6Co2Nd6 ribbons was only detected at high heating rates (20–40 K/min), in contrast to the Al86Ni4Co4Nd6 ribbons.
The results obtained for the alloys containing 6 at % Ni and 2 at % Co differ significantly for the neodymium- and samarium-containing ribbons. In addition to the fact that no glass transition was detected in the samarium-containing material, the first stage of its crystallization was shifted to lower temperatures by almost 46 K (at a heating rate of 10 K/min). Moreover, the heat effect in the first stage of crystallization decreases by almost three times. At the same time, these ribbons are similar in the nature of the second stage of crystallization (its temperature is 11 K higher in the case of the samarium-containing material). The peak temperature of the third stage of crystallization in the samarium-containing ribbons is higher than in the neodymium-containing ribbons (at any heating rate). It should be especially emphasized that, in this case, the heat effect in the samarium-containing material is considerably larger. In addition, at all heating rates the Al86Ni6Co2Sm6 alloy was found to have a fourth peak, at temperatures near 730 K, in contrast to the other samples. It seems likely that not only does a combination of 6 at % Ni, 2 at % Co, and 6 at % Sm considerably decrease the thermal stability of the amorphous state of the alloy, but it also stimulates an additional stage of crystallization.
At all heating rates, the glass transition temperature (Tg) is well-defined for the Nd-containing materials, which is atypical of amorphous aluminum-based alloys. The glass transition of the Sm-containing alloy with 4% Ni + 4% Co was only detected at high heating rates (20–40 K/min). This result correlates with previously reported DSC data for samarium-containing ternary alloys, whose Tg was never determined at a heating rate of 10 K/min [9–14].
The activation energy (Ep) for each stage of crystallization was evaluated by the Kissinger method [15], according to which
where Tp is the peak temperature, v is the heating rate, and R is the gas constant.
The calculation results are presented in Fig. 4 and Table 2.
The activation energy was determined to be E1 = 439 kJ/mol for the first stage of crystallization in the Al86Ni4Co4Nd6 alloy, E2 = 240 kJ/mol for the second stage, and E3 = 208 kJ/mol for the third stage. Replacing Nd by Sm increases E1 to 408 kJ/mol. The activation energy increases by more than 100 kJ/mol for the second stage of crystallization and by more than 60 kJ/mol for the third stage. The addition of cobalt as a second transition metal considerably increases the activation energies for the first and second stages of crystallization in comparison with the values obtained by Illekova et al. [10] for an Al89Ni6Sm5 ternary alloy.
Reducing the amount of Co from 4 to 2 at % reduces the activation energy for the first stage of crystallization to 353 kJ/mol, but increases the activation energies for the second and third stages to 339 and 216 kJ/mol, respectively.
Increasing the percentage of nickel in the samarium-containing alloys reduces the activation energy for the first stage of crystallization by almost a factor of 1.5, but raises the activation energy for the second stage of crystallization to 454 kJ/mol. The activation energy for the third stage of crystallization drops by 40 kJ/mol. The activation energy for the samarium-containing alloy with 6 at % Ni and 2 at % Co is substantially lower (by more than 90 kJ/mol for the first stage of crystallization) than that for the neodymium-containing alloy.
To identify the phases forming in different stages of crystallization, the ribbons were annealed for a short time, following which their structure was examined by X-ray diffraction. The ribbons were annealed during continuous heating at a rate of 10 K/min, followed by rapid cooling. The highest annealing temperatures were determined from the DSC results and corresponded to the first, second, and third peaks in the DSC curves of the alloys. The X-ray diffraction results for the Al86Ni6Co2Sm6 sample are presented in Fig. 5.
Pure aluminum was found to precipitate in the first stage of crystallization of the Al86Ni6Co2Sm6 alloy (Fig. 5, 570-K scan). The large width of the observed peaks in both the diffraction pattern and DSC curve of this sample suggests that aluminum precipitated in the form of nanoparticles. We observed the formation of the intermetallic phase Al9Co2 in the second stage of crystallization and the metastable compound Al4Sm (or possibly Al11Sm3 with a distorted lattice) in the third stage. At a temperature near 738 K, the metastable intermetallic phase Al4Sm converted into Al3Sm, a stable compound with a rhombohedral lattice (Fig. 5, 773-K scan).
On the whole, the Al86Ni4Co4Sm6 alloy exhibits similar crystallization behavior, but there are some distinctive features. First, the precipitation of aluminum nanoparticles in the first stage of crystallization begins at a 60-K lower temperature in comparison with the Al86Ni6Co2Sm6 alloy. Second, in the third stage the stable intermetallic phase Al3Sm with a cubic lattice is formed at once. Subsequent heating of the material is not accompanied by any heat effects. After heating to 700 K and cooling to room temperature, the material consisted of Al, Al9Co2, and cubic Al3Sm.
The X-ray diffraction results for the Al86Ni4Co4Nd6 alloy are presented in Fig. 6. Like in the case of the samarium-containing alloy, essentially pure aluminum precipitates in this alloy in the first stage of crystallization. Judging from the shape of the peaks in the X-ray diffraction pattern and DSC curve of this alloy, the pure aluminum particles in it are considerably larger than the aluminum nanoparticles precipitating in the samarium-containing alloys. In the second and third stages, the stable intermetallic compounds Al9Co2, Al5Co2, and Al3Nd are formed, persisting to room temperature. It is worth noting that, after heating to 700 K, we observed reflections corresponding to the AlNd3 compound, even though the existence of this compound at 6% neodymium appears unlikely. It is quite possible that there was a small amount of a ternary compound whose X-ray diffraction pattern was not represented in existing databases. Further investigation is needed to resolve this issue.
Thus, the crystallization onset temperatures and activation energies obtained here provide conclusive evidence that the Al86Ni4Co4Nd6 alloy has a broader stability range of its amorphous phase and is more thermally stable in comparison with the samarium-containing alloy. The 4% Ni + 4% Co combination is preferable to the 6% Ni + 2% Co combination for amorphous phase stabilization.
Figure 7 shows typical resistivity curves of the Al86Ni4Co4Sm6 and Al86Ni6Co2Sm6 alloys and a previously studied Al86Ni8Sm6 sample [8].
Relative resistivity curves of the Al86Ni8Sm6 [8], Al86Ni4Co4Sm6, and Al86Ni6Co2Sm6 alloys.
The resistivity of the amorphous alloys was found to be rather high, at a level of 120 μΩ cm. It is a weak function of temperature and has a negative temperature coefficient for all of the alloys. During crystallization, the resistivity decreases by more than a factor of 2, passing through three stages (their temperatures are identical to those evaluated from the DSC data to within ±3 K). It should be emphasized that, in the case of the samarium-containing ribbons, replacing 8 at % Ni by 6 at % Ni + 2 at % Co extends the temperature range of the amorphous state by 30 K (Tx1 shifts from 439 to 468 K). Further increasing the amount of Co to 4 at % extends the temperature range of the amorphous state by 100 K (Tx1 shifts from 439 to 535 K). Note that anomalous R(T) behavior was also observed at T\( \simeq \) 730 K.
Resistivity curves of the neodymium-containing alloy with 4 at % Ni and 4 at % Co have a number of distinctive features as compared to the samarium-containing alloy. The second stage of the decrease in resistivity during crystallization occurs in a wide temperature range (the resistivity gradually decreases with increasing T), whereas the third stage is represented by a small segment of the curve compared to the second stage. Above 650 K, the resistivity rises linearly with increasing temperature. No anomalies were detected near 730 K.
The anomalous R(T) behavior around 730 K in the case of the samarium-containing alloys is attributable to the cubic-to-rhombohedral phase transformation of the intermetallic compound Al3R. Such polymorphic transformations are typically not accompanied by considerable heat effects, but show up in temperature dependences of electron-sensitive properties [16].
The observed decrease in resistivity with increasing temperature for the amorphous phase can be accounted for as follows: Heating of amorphous Al–TM–REM alloys is known to cause phase separation well below the crystallization temperature. The effect was discovered by Gangopadhyay et al. [17] and was later confirmed many times for a variety of alloys (see, for example, Radiguet et al. [18] and Abrosimova et al. [19]). The formation of low-resistivity microregions consisting of essentially pure aluminum, whose size considerably exceeds interatomic distances, in a disordered amorphous matrix leads to a reduction in the total resistivity of the alloy. The larger the volume fraction of such microregions, the lower the resistivity of the material. When the crystallization of the ribbon reaches completion, its resistivity passes through a minimum and then begins to rise with increasing temperature, which is characteristic of crystalline alloys. This issue was addressed in detail previously [8].
Thus, the present resistivity data confirm that the neodymium-containing amorphous alloys are more thermally stable than the samarium-containing alloys and that the 4% Ni + 4% Co combination is more preferable than the 6% Ni + 2% Co combination for amorphous phase stabilization.
CONCLUSIONS
The present DSC and resistivity data demonstrate that the amorphous Al–Ni–Co–Nd(Sm) alloys have a broader temperature range of an amorphous state (by 100 K) than do Ni- or Co-containing ternary alloys and exhibit more complex crystallization behavior. Neodymium is more preferable than samarium for improving the glass-forming ability of these alloys, and the 4% Ni + 4% Co combination is better than 6% Ni + 2% Co.
ACKNOWLEDGEMENT
This work was supported by the projects VEGA 2/0082/17 and APVV-15-0049. B. Rusanov gratefully acknowledges the fellowship of the Slovak Academic Information Agency (SAIA).
REFERENCES
Inoue, A., Ohtera, K., Tsai, A.P., and Masumoto, T., Aluminum-based amorphous alloys with tensile strength above 980 MPa (100 kg/mm2), Jpn. J. Appl. Phys., 1988, vol. 27, pp. L479–L482.
Gloriant, T. and Greer, A.L., Al-based nanocrystalline composites by rapid solidification of Al–Ni–Sm alloys, Nanostruct. Mater., 1998, vol. 10, pp. 389–396. https://doi.org/10.1016/S0965-9773(98)00079-8
Triveco Rios, C., Suricach, S., Bary, M.S., et al., Glass forming ability of the Al–Ce–Ni system, J. Non-Cryst. Solids, 2008, vol. 354, pp. 4874–4877.
Ouyang, Y., Zhang, J., Chen, H., Liao, S., and Zhong, X., Crystallization study of amorphous Al82Fe5Ni5Ce8 alloy, J. Alloys Compd., 2008, vol. 454, pp. 359–363.
Botta, W.J., Triveno Rios, C., SaLisboa, R.D., et al., Crystallization behaviors of Al-based metallic glasses: compositional and topological aspects, J. Alloys Compd., 2009, vol. 483, pp. 89–93.
Li, C.L., Wang, P., Sun, S.Q., Voisey, K.T., and McCartney, D.G., Corrosion behaviour of Al86.0Co7.6Ce6.4 glass forming alloy with different microstructures, Appl. Surf. Sci., 2016, vol. 384, pp. 116–124. https://doi.org/10.1016/j.apsusc.2016.04.188
Vlasak, G., Duhaj, P., Patrasova, H., and Svec, P., Apparatus for thermal dilatation and magnetostriction measurements of amorphous ribbons, J. Phys. E, Sci. Instrum., 1983, vol. 16, pp. 1203–1207.
Sidorov, V., Svec, P., Svec, P., Sr., Janickovic, D., Mikhailov, V., Sidorova, E., and Son, L., Electric and magnetic properties of Al86Ni8R6 (R = Sm, Gd, Ho) alloys in liquid and amorphous states, J. Magn. Magn. Mater., 2016, vol. 408, pp. 35–40. https://doi.org/10.1016/j.jmmm.2016.02.021
Gich, M., Gloriant, T., Surinach, S., Greer, A.L., and Baro, M.D., Glass forming ability and crystallisation processes within the Al–Ni–Sm system, J. Non-Cryst. Solids, 2001, vol. 289, pp. 214–220. https://doi.org/10.1016/S0022-3093(01)00650-0
Illekova, E., Duhaj, P., Mrafko, P., and Svec, P., Influence of Pd on crystallization of Al–Ni–Sm-based ribbons, J. Alloys Compd., 2009, vol. 483, pp. 20–23. https://doi.org/10.1016/j.jallcom.2008.08.106
Sun, F. and Gloriant, T., Primary crystallization process of amorphous Al88Ni6Sm6 alloy investigated by differential scanning calorimetry and by electrical resistivity, J. Alloys Compd., 2009, vol. 477, pp. 133–138. https://doi.org/10.1016/j.jallcom.2008.10.021
Zhang, Y., Warren, P.J., and Cerezo, A., Effect of Cu addition on nanocrystallisation of Al–Ni–Sm amorphous alloy, Mater. Sci. Eng., A, 2002, vol. 327, pp. 109–115. https://doi.org/10.1016/S0921-5093(01)01888-3
Gangopadhyay, A.K. and Kelton, K.F., Effect of rare-earth atomic radius on the devitrification of Al88RE8Ni4 amorphous alloys, Philos. Mag. A, 2000, vol. 80, pp. 1193–1206. https://doi.org/10.1080/01418610008212110
Revesz, A., Varga, L.K., Nagy, P.M., Lendvai, J., and Bakonyi, I., Structure and thermal stability of melt-quenched Al92 –xNi8(Ce,Sm)x alloys with x = 1, 2 and 4, Mater. Sci. Eng., A, 2003, vol. 351, pp. 160–165. http://dx.doi.org/ 00823-7https://doi.org/10.1016/S0921-5093(02)
Kissinger, H.E., Reaction kinetics in differential thermal analysis, Anal. Chem., 1957, vol. 29, pp. 1702–1706.
Sidorov, V., Petrova, S., Svec, P., Sr., et al., Crystallization of Al–Co–Dy(Ho) amorphous alloys, Eur. Phys. J. Spec. Top., 2017, vol. 226, pp. 1107–1113. https://doi.org/10.1140/epjst/e2016-60226-x
Gangopadhyay, A.K., Croat, T.K., and Kelton, K.F., The effect of phase separation on subsequent crystallization in Al88Gd6La2Ni4, Acta Mater., 2000, vol. 48, pp. 4035–4043. https://doi.org/10.1016/S1359-6454(00)00196-8
Radiguet, B., Blavette, D., Wanderka, N., Banhart, J., and Sahoo, K.L., Segregation-controlled nanocrystallization in an Al–Ni–La metallic glass, Appl. Phys. Lett., 2008, vol. 92, paper 103 126. https://doi.org/10.1063/1.2897303
Abrosimova, G., Aronin, A., and Budchenko, A., Amorphous phase decomposition in Al–Ni–RE system alloys, Mater. Lett., 2015, vol. 139, pp. 194–196. https://doi.org/10.1016/j.matlet.2014.10.076
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the 16th International IUPAC Conference on High Temperature Materials Chemistry (HTMC-XVI), July 2–6, 2018, Yekaterinburg, Russia.
Rights and permissions
About this article
Cite this article
Rusanov, B.A., Sidorov, V.E., Svec, P. et al. Crystallization Behavior and Resistivity of Al–Ni–Co–Nd(Sm) Amorphous Alloys. Inorg Mater 56, 14–19 (2020). https://doi.org/10.1134/S0020168519120112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168519120112