Abstract
Using fluorescence spectroscopy, the two-photon absorption cross sections of aqueous solutions of the styryl dye trans-4-[4-(dimethylamino)styryl]-1-methylpyridinium iodide (DASPI) and its inclusion complexes with cucurbit[n]urils (CB[n] n = 6–8) have been measured. A nonmonotonic dependence of the cross section on the excitation wavelength and on the cavitand cavity size has been revealed. Compared to the free dye, a sevenfold increase in the two-photon absorption cross section has been observed in DASPI inclusion complexes with CB[8] at an excitation wavelength of 980 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In 1931, M. Göppert-Mayer theoretically predicted the phenomenon of two-photon absorption (hereinafter referred to as TPA) [1], and this nonlinear optics effect has been intensively studied both theoretically and experimentally for almost a hundred years, largely due to the fact that two-photon absorption is widely used in various physics, chemistry, biomedicine, etc. applications. In this regard, a large number of publications have recently appeared devoted to the study of the relationship between the efficiency of two-photon absorption and the chemical structure of organic fluorophores (see, for example, [2] and references therein).
Two-photon absorption with detection of excited luminescence is widely used in three- and two-dimensional scanning of objects with recording two-photon excited luminescence (hereinafter referred to as TPEL) and makes it possible to observe objects with high spatial resolution or, in other words, to create nonlinear optical microscopes that are widely used in biological and medical research [3–5] and appear to be useful in other areas where high spatial resolution is required or single-photon luminescence excitation is impossible.
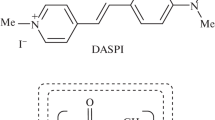
Another important example of the use of the TPA phenomenon is two-photon polymerization [6], where organic molecules with a large two-photon absorption cross section are required as initiators. Recently, Y.-C. Zheng et al. [7] used complexes of 3,6-bis[2-(1-methylpyridinium)vinyl]-9-pentylcarbazole diiodide with the cavitand cucurbit[7]uril (CB [7]) as a photoinitiator for two-photon polymerization in the aqueous phase, the presence of which increases the absorption cross section by approximately five times. The photoinitiator used in this work was a divalent molecular cation consisting of two identical subunits of close analogues of the typical styryl dye trans-4-[4-(dimethylamino)styryl]-1-methylpyridinium iodide (DASPI), the structure of which is shown in Fig. 1. Subunits linked by styryl tails are capable of forming an inclusion complex with CB [7]. The effect of complexation on two-photon absorption was observed apparently for the first time in the present study, and the mechanism of this effect remains unclear.
Cucurbit[n]urils (CB[n]) are hollow macrocyclic cavitands consisting of n glycoluril units (usually n = 5−8) bridged by pairs of methylene groups (see Fig. 1) [8]. At the ends of cucurbituril series molecules framing the entrances (portals) into the cavity, there are oxygen atoms of carbonyl groups that carry a partial negative charge. This promotes the binding of cucurbiturils with organic cations of suitable size, resulting in the formation of host–guest inclusion complexes.
Encapsulation of a “guest” molecule into the cucurbituril cavity, as a rule, leads to significant changes in the photophysical properties of the “guest” [9], in particular, to an increase in fluorescence intensity.
A feature of the molecular cations of styryl dyes is that they are intramolecular donor–acceptor systems A+–π–D, where the pyridinium unit A+ is an electron acceptor and dimethylaminophenyl D is a donor, linked by a π-electron conjugated bridge. The positive charge, which in the ground state is localized on the acceptor pyridinium group, moves toward the donor dimethylanilino group upon excitation [10]. It is known that there is a correlation between the nonlinear properties of a molecule and intramolecular charge transfer. Increasing the π-conjugation length, when single bonds alternate with double bonds, leads to an increase in the two-photon absorption cross section of the fluorophore.
This paper presents results on the effect of the cucurbituril cavity size on the two-photon absorption cross section of inclusion complexes using the example of DASPI as a relatively simple model molecule.
EXPERIMENTAL
Millipor Simplicity water was used to prepare the solutions. Cucurbiturils CB[6], CB[7], and CB[8] and the styryl dye trans-4-[4-(dimethylamino)styryl]-1-methylpyridinium iodide (DASPI) (all from Sigma-Aldrich) were used without further purification. The structural formulas of the molecules used in the study are shown in Fig. 1. One-photon absorption spectra were recorded using a Shimadzu UVmini 1240 spectrophotometer, and one-photon fluorescence spectra were recorded with a Fluorolog 3 Tau spectrofluorometer.
The fluorescence quantum yield was determined according to the known fluorophore standard method. The styryl dye 4-[(E)-2-(3,4-dimethoxyphenyl)-1-ethylpyridinium perchlorate], whose quantum yield is 0.026, was used as a reference sample [11].
The effect of cucurbiturils on the DASPI fluorescence intensity under two-photon excitation was studied on an optical bench that allows recording luminescence spectra and measuring the cross section of two-photon excitation of dye solutions in comparison with a reference sample in the wavelength range 750–1000 nm [12]. The main laser source of the bench is a Coherent Mira HP ultrafast titanium : sapphire oscillator generating femtosecond pulses with an energy of up to 40 nJ at wavelengths from 750 to 940 nm with a repetition rate of 78 MHz. Most of the radiation power was directed to synchronous pumping of a parametric pulse generator on a potassium titanyl phosphate crystal with a periodic domain structure, which made it possible to generate ultrashort pulses tunable in wavelength from 970 to 1550 nm. The dynamics of the two-photon response of the DASPI dye to the presence of cucurbiturils was studied at central pumping wavelengths of 790 and 980 nm. Pulse durations were measured using frequency-resolved optical gating, which were 110 and 130 fs at wavelengths of 790 and 980 nm, respectively. Laser radiation was introduced into an assembled microscope equipped with a Olympus XLUMPLFLN 20x/1.00 high-aperture IR lens with water immersion for florescence excitation and collection. Fluorescence was isolated by a system of dichroic mirrors and filters, after which it was directed to the light guide input of an Ocean Optics HR4000 compact spectrometer. The laser radiation power was up to 30 mW at the sample focus, and no signal degradation was observed during the measurements. To determine the absolute brightness of the two-photon response of DASPI, the recorded signal was compared with the reference signal obtained from a solution of rhodamine 6G in ethanol with a concentration of 9 × 10−6 mol/L, which has known nonlinear optical properties at these wavelengths and an overlapping luminescence spectrum with DASPI [13–15]. The dye solution was placed in a spherical recess on a microscope slide. The thickness of the dye solution in the meniscus of the microscope slide was the same in all experiments due to the fixed position of the slide on the microscope platform and was approximately 0.5 mm. The signal depended weakly on the lens focus position and was recorded from a depth of 200 µm from the surface through a 170-µm thick cover glass. The exposure time on the spectrometer was set from 20 to 400 ms depending on the brightness of the signal.
RESULTS AND DISCUSSION
To estimate the relative value of the two-photon absorption cross section, it is convenient to use the measurement of fluorescence excited by two-photon absorption [16]. The number of photons absorbed per unit time is given by
where σ2 is the two-photon absorption cross section, c is the dye concentration, and V is the irradiated volume. By definition, the number of emitted photons is given by Eq. (2):
Here φ is the fluorescence quantum yield.
With a decrease in the cavitand cavity size, which is determined by the number of glycoluril units n, the fluorescence quantum yields of the DASPI–CB[n] complexes upon one-photon excitation sharply increase (their values are presented in Table 1). The increase in fluorescence is attributed to the hindrance of intramolecular rotations of the dye cation during the formation of an inclusion complex [17].
Figure 2 shows values of brightness, i.e. the product of the two-photon absorption cross section and the fluorescence quantum yield, for free DASPI and its complexes with cucurbiturils CB[6], CB[7], and CB[8] upon excitation at 790 and 980 nm. Treating these data with the use of Eq. (2), we can determine the two-photon absorption cross section under the assumption that the fluorescence quantum yield does not depend on the method of generating the electronically excited state. The values of the two-photon absorption cross section are presented in Table 1.
The table shows that the magnitude of the two-photon absorption of DASPI depends on the excitation wavelength; noticeably greater at 980 than at 790 nm. In both cases, a nonmonotonic dependence on the cucurbituril size with a maximum for CB[8] was revealed. In the case of CB[8] excited at 980 nm, the absorption cross section increases by a factor of 7 compared to free DASPI.
Thus, the formation of supramolecular complexes of chromophores with cucurbiturils, with a successful choice of the cavitand size, can be an effective tool for increasing the two-photon cross section of the “guest” molecule.
REFERENCES
Göppert-Mayer, M., Ann. Phys., 1931, vol. 401, p. 273.
Collini, E., Phys. Chem. Chem. Phys., 2012, vol. 14, p. 3725.
Tsai, T.-H., Lin, C.-Y., Tsai, H.J., Chen, S.Y., Tai, S.P., Lin K.H., and Sun, C.-K., Opt. Lett., 2006, vol. 31, no. 7, p. 930.
Wiedenmann, J., Oswald, F., and Nienhaus, G.U., IUBMB Life, 2009, vol. 61, no. 11, p. 1029.
Chudakov, D.M., Matz, M.V., Lukyanov, S., and Lukyanov, K.A., Physiol. Rev., 2010, vol. 90, p. 1103.
Wloka, T., Gottschaldt, M., and Schubert, U.S., Chem. Eur. J., 2022, vol. 28, p. e202104191.
Zheng, Y.-C., Zhao, Y.-Y., Zheng, M.-L., Chen, S.-L., Liu, J., Jin, F., Dong, X.-Z., Zhao, Z.-S., and Duan, X.-M., ACS Appl. Mater. Interfaces, 2019, vol. 11, p. 1782.
Lee, J.W., Samal, S., Selvapalam, N., Kim, H.-J., and Kim, K., Acc. Chem. Res., 2003, vol. 36, p. 621.
Dsouza, R.N., Pischel, U., and Nau, W.M., Chem. Rev., 2011, vol. 111, p. 7941.
Ivanov, D.A., Svirida, A.D., and Petrov, N.Kh., High Energy Chem., 2022, vol. 56, no. 3, p. 149.
Ivanov, D.A., Petrov, N.Kh., Nikitina, E.A., Basilevsky, M.V., Vedernikov, A.I., Gromov, S.P., and Alfimov, M.V., J. Phys. Chem. A, 2011, vol. 115, p. 4505.
Lanin, A.A., Chebotarev, A.S., Pochechuev, M.S., Kelmanson, I.V., Kotova, D.A., Bilan, D.S., Ermakova, Y.G., Fedotov, A.B., Ivanov, A.A., Belousov, V.V., and Zheltikov, A.M., J. Biophoton., 2019, vol. 13, no. 3, p. e201900243.
Chebotarev, A.S., Lanin, A.A., Raevskii, R.I., Kostyuk, A.I., Smolyarova, D.D., Bilan, D.S., Savitskii, I.V., Fedotov, A.B., Belousov, V.V., and Zheltikov, A.M., J. Raman Spectrosc., 2021, vol. 52, p. 1552.
Makarov, N.S., Drobizhev, M., and Rebane, A., Opt. Express, 2008, vol. 16, p. 4029.
Reguardati, S., Pahapill, J., Mikhailov, A., Stepanenko, Y., and Rebane, A., Opt. Express, 2016, vol. 24, p. 9053.
Xu, Ch. and Webb, W.-W., J. Opt. Soc. Am. B: Opt. Phys., 1996, vol. 13, p. 481.
Svirida, A.D., Ivanov, D.A., Petrov, N.Kh., Vedernikov, A.V., Gromov, S.P., and Alfimov, M.V., High Energy Chem., 2016, vol. 50, no. 1, p. 21.
Funding
The work was supported by the Russian Science Foundation, project no. 22-23-00234.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated by S. Zatonsky
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Petrov, N.K., Ivanov, A.A., Ivanov, D.A. et al. Increase in the Two-Photon Absorption Cross Section of Styryl Dye in Supramolecular Complexes with Cucurbiturils. High Energy Chem 58, 302–305 (2024). https://doi.org/10.1134/S0018143924700140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143924700140