Abstract—The efficiency of phosphorylation in samples of isolated rat liver mitochondria in incubation media with variable isotopic composition at deuterium contents of 6, 60, 90, 120, 150 ppm, as well as 0.1, 1 and 2%, was studied. In media with a low deuterium content (up to 60 ppm), a monotonic decrease in the ADP/O ratio was observed, while at 6 ppm its value was the same as at 60 ppm. The generation of superoxide anion by complex I, II, and III of the mitochondrial respiratory chain was also investigated. For all studied modes of mitochondrial functioning using respiratory chain inhibitors, the generation of superoxide anion was independent of the deuterium concentration in the incubation medium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
It is long established that respiration of intact mitochondria, submitochondrial particles, and isolated electron transport complexes is inhibited in D2O [1, 2]. The strongest isotopic effects in concentrated heavy water, amounting to approximately 50%, are observed in processes associated with phosphorylation, i.e., in the cytochrome region. Natural water is known to contain an approximately 0.015% (or 150 ppm) level of deuterium atoms on average. In nature, this value varies in different sources as a result of fractioning deuterium isotopes during phase transitions of water, as well as during its adsorption. Under equilibrium at the triple point of water, the lowest content of deuterium will be in the vapor phase and the highest content will be in the solid phase, but these effects are relatively small. The water that is most deuterium depleted is observed in the precipitation at the South Pole, where the D/H ratio = 0.0089 at % (89 ppm), and the most enriched water is found in closed basins of the arid zone, where D/H = 0.0178 at % (178 ppm) [3]. Considering that the magnitude of the isotopic effect in respiration of mitochondria will linearly depend on the concentration of deuterium in aqueous media, it is difficult to expect significant effects with natural variations of deuterium and even if deuterium is completely absent in water. Nevertheless, as far back as the 1930s, significant isotopic effects in living organisms in water containing high deuterium contents of up to 0.06% were found [4]. The activating effect of melt water from snow on living organisms caused by a reduced concentration of deuterium was first shown in [5, 6], where it was also proved that the observed effects are precisely associated with a reduced concentration of deuterium. A review of the author’s early papers and papers on this subject can be found in [7], and later papers are in [8]. There has been no adequate explanation of the effects that accompany variations in the isotope composition of water in the region of low deuterium concentrations. The isotope resonance hypothesis is worth noting, which includes consideration of the main isotopes of biomacromolecules: carbon, deuterium, oxygen, and nitrogen [9]. It is impossible to isolate only one organelle or any one process that is responsible for the unexpectedly large effects observed in living organisms with a relatively small change in the deuterium content in the aqueous medium; however, mitochondria, which are the main suppliers of energy in the form of ATP and generators of reactive oxygen species, are clear candidates for these effects.
MATERIALS AND METHODS
Female Wistar rats with a weight of approximately 200 g were used in the experiments. Mitochondria isolated from the livers of female rats according to the standard protocol were used [10]. During the experiment, the mitochondria were stored in a thick suspension (protein concentration of approximately 150 mg/mL) on ice in Eppendorf tubes with a small amount of washing medium (210 mM mannitol, 40 mM sucrose, 5 mM HEPES, 0.5 mM EDTA, pH 7.4). The measurement medium (320 mOsm, pH 7.4) contained 285 mM mannitol, 13 mM KCl, 3 mM HEPES, 0.25 mM EDTA, 1 mM MgCl2. The temperature was stabilized at 30°С using a F25 thermostat (JULABO GmbH, Germany) with an accuracy of 0.1°С. The rate of mitochondrial respiration was measured by the polarographic method in a 600 mL cell using a Clark oxygen electrode (Strathkelvin Mitocell MT200, Oxygen Meter 782; Cole-Parmer, United States). Three μL of mitochondrial suspension was added to the cell. Phosphorylation was measured under conditions of succinate dehydrogenase (complex II) functioning, in the presence of 0.5 μM rotenone and 3 mM succinic acid in the cell of the polarograph. To trigger phosphorylation, 160 μM ADP and 250 μM phosphate were added.
The ADP/O parameter, which characterizes the efficiency of the respiratory chain, was calculated as the ratio of the molar amount of added ADP to the amount of atomic oxygen absorbed by the mitochondrial suspension during the complete phosphorylation of added ADP. The maximum theoretical value of this parameter under the conditions of complex II functioning is equal to two. The lower value of ADP/O in our experiments may be due to the presence of fatty acids (natural uncouplers) in the mitochondrial preparation, as well as the use of low (below the saturation of enzymes) concentrations of phosphate and ADP, which limited the rate of phosphorylation.
The duration of one polarogram record was 8 min. During this period, mitochondria had no time to experience noticeable oxidative damage as shown by the constant respiration rate during phosphorylation in the control experiments. Immediately after the isolation of mitochondria, the first series of records from 6 ppm deuterium to high concentrations was started. After the completion of the first series of experiments, the measurements were repeated in the same order. The time between records of the corresponding polarograms of the first and second series was approximately 2 h. The measurements on the mitochondria from one rat were repeated twice. In total, two experiments were performed, i.e. four measurements were performed for each concentration of deuterium.
The H2O2 formation was measured using Ampex Red dye as described previously [11]. Next, 120 mM KCl, 10 mM HEPES-tris, 2 mM K2HPO4 at pH 7.4, 10 μM Amplex Red and 5 U/mL horseradish peroxidase were added to the incubation medium of mitochondria. The fluorescence intensity was recorded with a FluoroMax instrument (Horiba Jobin Yvon GmbH, Germany) at a wavelength of 600 nm under an excitation wavelength of 530 nm. Standard solutions of hydrogen peroxide were used for the calibration. Sequential inhibition of mitochondrial respiratory chain complexes was carried out in the experiment: first 1 μM rotenone (complex I inhibitor) was added, then 1 μM antimycin (N-center inhibitor of complex III of the respiratory chain) was added, and finally 1 μM myxothiazole (P-center inhibitor of complex III of the respiratory chain) was added.
To prepare solutions of the incubation medium, water from the Almaz company (Moscow) with a specific conductivity of 3.61 μS cm, containing deuterium isotopes (D = 4 ppm) and heavy oxygen (18O = 849 ppm and 17O = 170 ppm), was used. Taking the dilution into account, mitochondrial suspensions were studied at reduced deuterium concentrations (6, 60, 90, and 120 ppm). The deuterium content in ordinary water was taken as 150 ppm. The increased deuterium content was provided by adding the necessary amount of heavy water (D2O at a concentration of 99.6%) to 1000, 10 000 ppm (1%), 20 000 ppm (2%).
RESULTS AND DISCUSSION
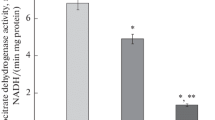
The results of the measurement of ADP phosphorylation by a suspension of mitochondria are presented in Fig. 1. It is seen that both a decrease and an increase of the content in water relative to its natural content led to a significant decrease in phosphorylation efficiency. In the series of decreasing deuterium concentrations of 150–120–90–60 ppm, a monotonic increase in the inhibition is observed. The phosphorylation efficiency at 6 ppm was at a minimum and did not differ from that at 60 ppm. With an increase in the concentration of deuterium from its natural content to 1%, the phosphorylation efficiency also decreased, but to a lesser extent. Under further increase in the concentration of deuterium to 2%, the activation of phosphorylation reaching the value in ordinary water was observed. The measurement of the rate of oxygen absorption yielded an average value of 22.5 μmol O2/min; however, the large dispersion (associated with the time elapsed after the isolation of mitochondria) at each concentration of deuterium in the samples did not allow the detection of isotopic effects in the medium in the range of concentrations used. The difference in the ADP/O ratio with the absence of significant differences in the phosphorylation rate and the value of respiratory control (the ratio of respiration rates under phosphorylation and in its absence) is due to the fact that the differences in oxygen consumption in samples with different deuterium concentrations were significant mainly at low ADP concentrations, which were achieved by the end of the phosphorylation process.
(a), The ADP/O parameter, which characterizes the efficiency of phosphorylation. Different series of experiments are indicated with different symbols. Line illustrates the dependence of the mean values. (b), A histogram illustrating the quantitative comparison of experimental data. Error margins characterize the standard deviation (N = 4 at 6 and 60 ppm and N = 6 at 150 ppm). The difference from the control at 6 and 60 ppm satisfies the significance level of 0.05; at 10 000 ppm it satisfies the significance level of 0.1.
It should be noted that phosphorylation efficiency measurements were carried out under conditions of a low phosphate concentration and a relatively low HEPES buffer concentration. It was previously shown that it is under such conditions that the formation of a fraction of nonequilibrium membrane-bound hydrogen ions, which is used for the synthesis of ATP, is observed [12]. This fraction of hydrogen ions significantly depends on the structural organization of water at the membrane surface; therefore, it can be most sensitive to the isotopic effect.
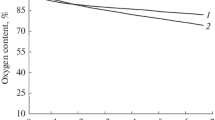
Typical examples of the results of hydrogen peroxide production measurements in the presence of inhibitors are presented in Fig. 2. Three sources that generate superoxide anion are known in the respiratory chain of mitochondria: complex I, which produces superoxide anion during reverse electron transfer from complex II; the P-center of complex III, which actively produces superoxide anion in the case of N-center blockade of the Q cycle; and complex II, which produces superoxide anion at the flavin center in the forward (from flavin to coenzyme Q) and reverse (from coenzyme Q to flavin) electron transfer reactions [13–15]. The rate of formation of hydrogen peroxide, in which superoxide anion was rapidly converted in mitochondria due to the functioning of superoxide dismutase, was experimentally recorded. It can be seen from Fig. 2 that the generation rate does not differ significantly in samples with different concentrations of deuterium in the medium: both during superoxide anion generation by complex I and complex III (the addition of antimycin A, a N-center inhibitor of complex III, was induced). The P-center inhibitor of complex III, myxothiazole, almost completely eliminated superoxide anion generation caused by antimycin A; however, differences in the generation of superoxide anion between media with different deuterium concentrations were also not observed in this mode (data not shown). In addition, inhibitory analysis during the functioning of complex II at low succinate concentrations (100 μM) in the presence of rotenone (an inhibitor that effectively blocks the reaction of both forward and reverse electron transfer in complex I) was performed. Under conditions of incomplete saturation of complex II, the rate of superoxide anion synthesis was higher than at 2 mM succinate; however, differences between samples with different deuterium concentrations were also not observed (data not shown). The results on the measurement of the rate of superoxide anion synthesis in samples of isolated mitochondria are completely consistent with the literature; however, in the measurement media with isotope compositions of the medium of 6, 60, 90, 120, 150 ppm, as well as 0.1 and 1%, hydrogen peroxide generation turned out to be the same with high accuracy (the discrepancy is not more than that observed between control experiments). Thus, we can conclude that a change in the concentration of deuterium did not affect the production of superoxide anion by complex I, II, and III of the respiratory chain under the experimental conditions.
Production of Н2О2 (formed from superoxide anion) by samples of isolated mitochondria at various concentrations of deuterium. The first part of the plots (approximately 5 min) corresponds to superoxide anion generation during the process of reverse electron transport on complex I. The second part of the plots (in the presence of rotenone and antimycin A) corresponds to the generation of superoxide anion by complex III.
These results on hydrogen peroxide generation differ from those published previously [16], where an approximately 2-fold activation of the maximum rate of Н2О2 generation by isolated rat liver mitochondria using water containing 52 ppm deuterium without using respiratory chain inhibitors was shown. It should be noted that our experiments (Fig. 2) were carried out under the conditions of succinate oxidation and the presence of ATP; this guaranteed a high membrane potential, which is as an important factor of ROS generation, especially in the case of reverse electron transfer from complex II to complex I. However, in experiments in which rotenone was present from the beginning of the experiment, we also failed to see isotopic effects. The comparison of the results obtained in this work and the literature results suggests that the isotopic effect of deuterium in the generation of hydrogen peroxide can occur under forward electron transfer on complex I (from NADH to ubiquinone); however, this assumption requires additional verification. It is worth noting that some enzymes of the Krebs cycle can act as a source of superoxide anion in mitochondria in addition to complexes of the respiratory chain. One could not exclude the effect of deuterium on their functioning.
In [16] the experimental group of rats was provided with drinking water with a deuterium content of 46 ppm for 28 days; the production of hydrogen peroxide by isolated mitochondria in the medium with normal deuterium content and reduced to 46 ppm was then analyzed. It was shown that mitochondria of the rat liver that were adapted to deuterium depleted water produced 35% more H2O2 in the same water than mitochondria of rats under normal conditions and tested in ordinary water. Qualitatively this effect is observed both with the addition of succinate and without the addition of a substrate due to internal metabolites. An important result, which the authors did not note, is that rat liver mitochondria that were adapted to light water but tested in ordinary water showed the same results as in the control. This means that the isotopic effects of light water are due to the action of water as a solvent and do not significantly affect systems of the living cell during the process of long-term adaptation of animals to water with a reduced isotopic composition. This result was recently indirectly confirmed in [18], in which the role of mitochondrial proteins in changes of the redox status of cell cultures placed in deuterium depleted medium was revealed by the latest methods of Ox-Red Proteomics during the analysis of 2935 proteins.
REFERENCES
S. A. Margolis, H. Baum, and G. Lenaz, Biochem. Biophys. Res. Commun. 25 (1), 133 (1966).
V. I. Lobyshev and L. P. Kalinichenko, Isotopic Effects of D2O in Biological Systems (Nauka, Moscow, 1978) [in Russian].
V. I. Ferronskii and V. A. Polyakov, Isotopy of the Earth’s Hydrosphere (Nauchnyi Mir, Moscow, 2009) [in Russian].
T. C. Barnes, Science 79 (2050), 370 (1934).
B. N. Rodimov, Sel’sk. Khoz. Sibiri, No. 7b, 66 (1961).
I. V. Toroptsev, B. N. Rodimov, A. M. Marshunina, in Problems in Radiobiology and Hematology (Tomsk State Univ., Tomsk, 1966), pp. 118–126 [in Russian].
V. I. Lobyshev, Aktual. Vopr. Biol. Fiz. Khim. 3 (3), 511 (2018).
A. Basov, L. Fedulova, M. Baryshev, and S. Dzhimak, Nutrients 11, 1903 (2019).
X. Xie and R. A. Zubarev, Sci. Rep. 5, 9215 (2015).
D. Johnson and H. Lardy, in Methods in Enzymology, ed. by R. W. Estbrook and M. E. Pullman (New York, Academic, 1967), Vol. 10, pp. 94–96.
A. A. Selin, N. V. Lobysheva, O. N. Vorontsova, et al., Bull. Exp. Biol. Med. 153 (1), 44 (2012).
S. A. Eremeev and L. S. Yaguzhinskii, Biochemistry (Moscow) 80 (5), 576 (2015).
M. D. Brand, Exp. Gerontol. 45, 466 (2010).
F. L. Muller, Y. Liu, and H. Van Remmen, J. Biol. Chem. 279, 49064 (2004).
C. L. Quinlan, A. L. Orr, I. V. Perevoshchikova, et al., J. Biol. Chem. 287, 27255 (2012).
I. A. Pomytkin and O. E. Kolesova, Byull. Eksp. Biol. Med. 142 (11), 514 (2006).
S. S. Dzhimak, A. A. Basov, N. N. Volchenko, et al., Dokl. Biochem. Biophys. 476, 323 (2017).
X. Zhang, M. Gaetani, A. Chernobrovkin, and R. A. Zubarev, Mol. Cell. Proteomics 18 (12), 2373 (2019).
ACKNOWLEDGMENTS
The authors thank L. S. Yaguzhinskii for useful discussions.
Funding
Part of this work (measurement of mitochondrial respiration) was financially supported by the Russian Foundation for Basic Research (project no. 19-04-00835\19)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All procedures for the maintenance and use of animals were in accordance with the standards of the International Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Additional information
Translated by D. Novikova
Rights and permissions
About this article
Cite this article
Lobysheva, N.V., Nesterov, S.V., Skorobogatova, Y.A. et al. The Functional Activity of Mitochondria in Deuterium Depleted Water. BIOPHYSICS 65, 272–276 (2020). https://doi.org/10.1134/S0006350920020128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350920020128