Abstract
The article proposes a hypothesis for the functioning of a diatom population under conditions of clearly defined water column stratification. It is assumed that cells in the upper illuminated water layer accumulate biomass based on photosynthesis. In the lower layer, which is rich in minerals, cells replenish their intracellular reserves. Movement from one layer to another allows cells to compensate for lack of resources. Sinking occurs as a result of sedimentation and lifting results from the release of carbon dioxide microbubbles during respiration. This hypothesis was used to construct an agent-based model of a diatom population. Calculations have shown that vertical movements maintain the existence of the population. Different regimes of surface illumination make it possible to obtain various distributions of cell numbers in the upper and lower water layers, which is consistent with the data of expeditionary observations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In recent decades, the ecosystems of Arctic regions have undergone significant transformations due to climate change and different anthropogenic impacts. Detailed studies of the ecosystem of the Arctic seas of Russia, which have been carried out in the last two decades during expeditions of the Institute of Oceanology, Russian Academy of Sciences, under the guidance of Academician M.V. Flint, have significantly expanded our knowledge of the ecological state of this region. The Kara and Laptev seas are mainly shallow shelf Arctic basins with a clearly defined density stratification of the water column as a result of a significant inflow of fresh water from the Ob, Yenisei, Lena, and other Siberian rivers. The two-layer, almost immiscible hydrophysical structures of waters of the Laptev Sea have a desalinated layer depth of 6–7 m; in the Kara Sea, they reach 15–20 m.
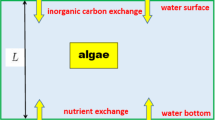
One of the most important components of a marine ecosystem is the phytoplankton community, which determines its primary production and the functioning of all subsequent links in the food chain. It is known that the basins of the Kara and Laptev seas have an extremely low total biological production [2, 8, 12]. Many authors see the cause of this phenomenon in the existence of two almost immiscible water layers: the upper desalinated layer deficient in biogenic elements and the lower layer, which is more saline and more enriched with nutrients [13, 25]. The upper layer of water is sufficiently well illuminated, while the light intensity in the lower layer is insufficient for photosynthesis. Diatoms (Thalassiosira nordenskioeldii, T. hyaline, Chaetoceros furcellatus, etc.) make up a significant part of the phytoplankton biomass in the Arctic seas in summer. In contrast to many other phytoplankton groups, diatoms do not have flagella and can not actively move between immiscible layers of the water column. This naturally poses the question: how can diatom populations maintain their existence in this system?
Indirect data on the production process and functional state of the photosynthetic apparatus of a phytoplankton community based on measurement of parameters of algal chlorophyll fluorescence can contribute to understanding the mechanisms of phytoplankton functioning in the given region. This approach makes it possible to determine the potential efficiency of photosynthesis and the dependence of the efficiency of photosynthesis on the degree of light intensity (the light curve of photosynthesis), determine the deficiency in mineral nutrients, and establish the degree of adaptation of algae to light intensity in the phytoplankton habitat.
The state of the photosynthetic apparatus of individual cells of large diatoms (Thalassiosira nordenskioeldii) from waters of the Kara and Laptev seas was assessed in the summer of 2018 during cruise 72 of the R/V Akademik Mstislav Keldysh, which was carried out as part of the long-term program “Ecology of Siberian Arctic Seas” [16]. This expedition revealed significant differences in the degree of adaptation of algae in the upper layers of the water column under conditions of relatively low salinity with a deficiency of mineral nutrients and algae at depths of more than 20 m, where the water has a high salinity and a favorable mineral composition for algae. Cells of algae in the upper water layer are adapted to a relatively high light intensity. They have effective protection against excess light. These cells can move to deeper layers with low light conditions; however, they cannot immediately adapt to new conditions. At the same time, the potential efficiency of algal photosynthesis, measured after preliminary adaptation in the dark for 2 h, proves to be quite high both in cells that were exposed to the upper horizon and in cells that were exposed to the lower horizon. This property of algae is characteristic of their growth in favorable conditions with no deficiency. It is well known that, under favorable conditions, many algal species can store mineral substances in an amount sufficient to maintain normal metabolism and subsequent division without experiencing any deficiency [3, 6]. In situ measurements of the chlorophyll content by the fluorimetric method demonstrate extremely diverse depth distributions [10]. The maximum chlorophyll content can be observed near the surface and at depths below the water density discontinuity or simultaneously in the upper and lower layers. During repeated measurements, the displacement of the vessel due to drift significantly changes the distribution profile (Fig. 1).
It can be assumed that the long-term existence of phytoplankton under such conditions is possible when cells move not only from the upper to the lower layer but also when cells return from the depth to the upper water layer. Thus, the authors of [4, 7] describe the vertical movement of diatom macrocolonies based on buoyancy regulated by gas bubbles. In most cases, we fail to observe such natural structures, since they can be destroyed during the selection and further preparation of phytoplankton samples for study. Academician V.I. Belyaev [1, 11] studied the effect of turbulence on phytoplankton photosynthesis based on the kinetic theory of macroscopic particles. However, he did not take into account the clear separation of the medium into layers with different characteristics. We tested the hypothesis of movement of individual cells and the assumption about the functioning of phytoplankton in a two-layer sea using the proposed mathematical model.
This paper presents an agent-based model of phytoplankton population dynamics, which reflects the features of functioning of diatom phytoplankton in some northern seas, in particular, the Kara Sea. In this region, the distribution of water salinity and density is determined by a large continental flow of river waters and ice melting. The distribution of salinity also determines the distribution of water density. In summer, the density of the upper layer (5–10 m) is very low and increases with a very sharp jump in depth. The water column is, as it were, divided into two layers: sunlight is available for microorganisms in the upper layer and necessary elements of mineral nutrition are available in the lower layer. Therefore, phytoplankton cells have to move from one layer to another to survive.
The following hypothesis about the behavior of a diatom cell is put forward. In the illuminated water layer, it accumulates biomass based on photosynthesis. In doing so, the cell increases its density by maintaining the volume based on the presence of solid silicon valves. As the density increases, the cell gradually sinks until its density reaches the level of density of more saline water. In this layer rich in minerals, the cell replenishes its intracellular reserves. Once in the absence of light, the cell begins to spend the accumulated carbohydrates on different metabolic processes, including respiration. The released carbon dioxide is retained around the cell in the resulting mucus sac. At the same time, the specific biomass of this “formation” (cell + “bag”) gradually decreases. Upon reaching the critical density value, the cell floats to the surface and the gas bubble collapses.
This model attempts to explain the mechanism of functioning of the phytoplankton community and draw the attention of phytoplanktonologists to the facts confirming or refuting the proposed hypothesis.
MODELING METHODS
The model was created in a specialized integrated NetLogo development environment [21].
General Characteristics of the Model
The computer population consists of a set of agent microalgae cells. The medium is a regular grid (Fig. 2). Each unit of the grid is characterized by two values: light intensity and nutrient content. The medium is divided into two layers: in the upper layer, the nutrient content is assumed to be equal to zero and the light intensity decreases with depth (vertically) according to the exponential law. In the lower layer, illumination is absent and nutrients are evenly distributed. It is assumed that the content of all “resources” is sufficient and does not change in a grid unit when consumed by agent cells of microalgae.
Interface of agent-based model. Right, model “scene” (vertical section of water column) divided into two layers, with randomly arranged agent cells. Left, agent counters, set values of model parameters, and graph of changes in total number of agent cells. Software settings make it possible to display graphs of time changes for all model counters, if necessary.
The population of algal cells is divided into two groups: algae cells located in the upper (ups) and lower (downs) layers of the medium. The state of each agent is described by two characteristics limited from above: density (specific biomass) and intracellular content of minerals. In addition, an agent cell has a state of adaptation (A) either to light or to darkness. The following actions are possible for each algae cell: increase/decrease in density; storage/consumption of minerals; division; death; transition from one group to another; movement; and the state of adaptation changes after the transition to another group in a certain period of time.
Cells of the upper group absorb light and increase the specific biomass due to photosynthesis; during this process they consume the intracellular content of minerals. Cells of the lower group extract minerals from the medium and store them in intracellular depots and reduce their density by consuming organic substances for different metabolic processes, including respiration.
Cells of the upper group move down when their density reaches the upper threshold value (\({{m}_{{\max }}}\)). Their vertical position in the lower layer is determined randomly. Cells of the lower group move up when their density decreases to the lower threshold value (\({{m}_{{\min }}}\)). As a result of the transition, the cell finds itself in the near-surface water layer. Within their layer, cells move in a random direction.
Functional dependences and parameter values used in the model are given in Table 1.
Parameters of the Medium
The light intensity I at each point of the upper layer of the model medium is determined by the Bouguer–Lambert–Beer law and depends on the I0 value, which corresponds to the illumination of the water surface, and on the vertical y coordinate.
Illumination is measured in relative units (r.u.). Illumination that is conditionally favorable for a phytoplankton cell (agent cell) is assumed to be equal to 1. The depth of the upper layer corresponds to 10 m.
The content of nutrients in the lower layer of the model medium can be set in accordance with the experimental values. At this stage of modeling, we believe that they are always in excess. This assumption is based on the possibility of permanent replenishment of mineral substances based on their supply from deep sea layers. The model time is measured in hours.
Agent Cell Parameters
The specific biomass of a cell varies from 1.022 g/cm3 (\({{m}_{{\min }}}\)) to 1.028 g/cm3 (\({{m}_{{\max }}}\)), which corresponds to the water density in the upper desalinated and lower saline layers of seawater.
The maximum intracellular content of mineral substances \({{q}_{{\max }}}\) is assumed to be 70 pg. Analysis of the literature data showed that this indicator could vary thousands of times depending on the species of microalgae and environmental conditions [5, 9, 14, 15, 17–20, 22–24]. The value of this parameter can then be set more accurately based on experimental data.
At the initial time, a certain number of agent cells (the same in each layer) with specified characteristics are generated in the model. The numerical experiment was carried out with an initial number of 100 and 1000 cells. The initial specific biomass in the upper and lower layers was equal to the minimum value and maximum possible value, respectively. The intracellular reserves of cells were set to be 70 pg in the upper layer and 50 pg in the lower layer.
Ups cells. The increase in the density (specific biomass, \(m_{i}^{u}\)) of an agent cell of the ups group depending on illumination I is described by the discrete logistic law. Subscript i indicates the current time (h). Parameter \(k_{m}^{{{\text{get}}}}\), which characterizes the rate of density increase, is selected so that this density increases monotonously and can reach its maximum value at surface illumination I0 = 1 r.u. in about 50 h (Fig. 3). When a cell of the ups group accumulates the maximum possible density (\({{m}_{{\max }}}\)), it moves to the downs group.
Increase in cell density \(m_{i}^{u}\) in upper layer of medium under conditions of surface illumination I0 = 1 r.u. Time measured in h. Calculation formula given in Table 1.
The loss of the intracellular reserve of minerals \(q_{i}^{u}\) is proportional to the current value and the coefficient of proportionality depends on illumination; the higher the illumination, the more rapid the consumption rate. The empirical dependence and parameter values are selected so that a cell in the upper layer loses about 15% of the quota at an illumination of I0 = 1 r.u. and about 35% at an illumination of I0 = 2 r.u.
The mortality of cells of the ups group is probabilistic in the model. When unfavorable conditions occur (illumination below 0.1 r.u. and depletion of intracellular reserves of mineral nutrition or extremely low group population), it is believed that the cell can live for an arbitrarily long time. This behavior can be correlated with a particular metabolic state of the cell (anabiosis). Otherwise, the probability of cell death is higher at high illumination I, a low value of quota \(q_{i}^{u}\), and a high group density \(N_{{{\text{cells}}}}^{u}\) (Fig. 4).
Dependence of probability of cell death \(p_{{{\text{death}}}}^{u}\) on level of illumination at \(N_{{{\text{cells}}}}^{u} = 2000\), \(q_{i}^{u} = 35\). Calculation formula given in Table 1.
Parameter A, which characterizes adaptation to environmental conditions, is assumed to be equal to 1 for ups cells. After the transition of a cell to the downs group, it changes for 0 after 4 h.
Cells of the downs group. The reduction of the density (specific biomass \(m_{i}^{d}\)) of an agent cell of the downs group is assumed to be proportional to its value at a given time with a value of the coefficient of proportionality \(k_{m}^{{{\text{loose}}}}\) so that the cell loses its density to a minimum value in 60 hours. When the density decreases to the minimum possible value, the cell of the downs group \({{m}_{{\min }}}\) moves to the ups group.
The accumulation of intracellular reserves of mineral nutrition \(q_{i}^{d}\) is described by a discrete logistic growth equation. The set value of parameter of rate \(k_{q}^{{{\text{get}}}}\) replenishes the reserves consumed by the cell at surface illumination I0 = 2 r.u. to a maximum level in 20 h (Fig. 5).
Accumulation of intracellular content of mineral substances \(q_{i}^{d}\) by agent cells in lower layer of medium. Time measured in h. Calculation formula is given in Table 1.
The possibility of division is given in the model only for the cells of the lower layer. It is implemented in cases when the cell has a sufficient reserve of mineral nutrition and a sufficient biomass. During the division, two daughter cells are formed from one mother cell; their density is the same as that of the mother cell and their intracellular quota is equal to half the quota of the mother cell. The density of 1.0249 g/cm3 (\(0.997{{m}_{{\max }}}\)) and the intracellular content of mineral substances at the level of 56 pg (\(0.8{{q}_{{\max }}}\)) were taken as the threshold values. They were selected so that the resulting daughter cells were viable within the rules specified in the model.
The mortality of cells in the lower layer is directly correlated with their division: every fourth cell capable of division dies.
Parameter A for downs cells is assumed to be equal to 0. After the transition of a cell to the ups group, it changes to 1 in 4 h.
RESULTS AND DISCUSSION
The key assumption behind the proposed model is that cells move between two water layers, each of this layers contains only one of the two resources necessary for life: sunlight or nutrient minerals. In the model experiment, the prohibition on the movement of agent cells between the ups and downs groups leads to the preservation of the minimum number of cells in the “anabiotic” state in the population, which is established by the rules of the model. Therefore, it is the vertical movement of cells that maintains the existence of the population.
Data of expeditionary studies indicate a high spatial and temporal heterogeneity of the chlorophyll content. Model experiments also demonstrate a variety of behaviors of the population of agent cells.
At the level of near-surface illumination I0 = 1 r.u. in the model experiment, the total number of cells periodically changes with an interval of about 55 h (Fig. 6a). At certain points of time the numbers of cells in ups and downs are equal (Fig. 6b).
When illumination increases by 2 times (I0 = 2 r.u.), the period of change in the total number of cells increases to 80 h (Fig. 7a). Excessive light conditions cause more frequent cell death. In addition, the accelerated consumption of intracellular mineral reserves in the sun leads to a longer period of their replenishment in the lower layer. As a result, the number of cells of the upper group varies more significantly, periodically decreasing to 0 (Fig. 7b). Such natural observations are sometimes recorded in expeditionary measurements (Fig. 1).
When the near-surface illumination decreases to 0.6 r.u., the period of change in the total number of cells increases to 150 h (Fig. 8a). The maximum numbers of the upper and lower cell groups replace each other (Fig. 8b).
The behavior of the model is significantly influenced by the parameter that sets the distance each cell moves in a random direction at every moment of time. Its strong increase can be correlated with the forced movement of phytoplankton cells under rough sea conditions. In addition, the presence of phytoplankton at a certain environment point can also be influenced by the factor of turbulence of different types. As a result, the “availability” of sunlight decreases. The model experiment demonstrates a decrease in the total number of cells (Fig. 9a) in comparison with the same experiment in the absence of significant cell movements (Fig. 6a). Higher surface illumination (I0 = 2 r.u.) leads to an increase in the total number of cells moving over long distances (Figs. 9c and 6c), since they prove to be less prone to photodegradation in this case. The dynamics of the cell numbers of ups and downs groups also changes: at near-surface illumination of 1 r.u., the maxima in the numbers of the upper and lower cells replace each other, which was characteristic of low illumination (Fig. 9b); at an illumination of I0 = 2 r.u., the number of lower cells exceeds that of upper ones (Fig. 9d).
Under natural conditions, the illumination of water surface varies greatly. In the numerical experiment, we set its periodic change: the values of 0.6, 1, and 2 r.u. replaced each other every 120 h for 100 model days (Fig. 10). Under such conditions, the total number of cells fluctuates with a period of about 150 h (Fig. 11a) and the distribution in the groups demonstrates the change of the dominance of the upper and lower cells (Fig. 11b).
All the model experiments showed that there were cells with A = 0 among the ups cells, for which parameter A should be equal to 1. This means that the group contains agent cells that moved to this group from another group no more than 4 h ago and are adapted to the conditions of the lower layer of the medium. Similarly, some of the cells are characterized by parameter A = 1 in the downs group, where they moved to from the ups group no more than 4 h ago. Figure 12 shows the proportion of cells with A = 0 in the ups group in the model experiment with a periodic change in near-surface illumination.
Thus, the data resulting from the agent-based model of a diatom population support the hypothesis of the existence of two groups of cells and the ability of the population to survive based on the movement of cells between these groups. By changing the conditions of water surface illumination in the model, we can obtain different distributions of cells in the groups, which are characteristic of expeditionary observations.
REFERENCES
V. I. Belyaev, Kinetic Theory of Systems Consisting of Macroscopic Particles and Its Application to the Problems of Ecology (Mor. Gidrofiz. Inst. Nats. Akad. Nauk Ukrainy, Sevastopol, 1998) [in Russian].
A. B. Demidov, S. V. Sheberstov, and V. I. Gagarin, “Estimation of annual Kara Sea primary production, Oceanology 58 (3), 369–380 (2018).
L. V. Il’yash, Extended Abstract of Doctoral Dissertation in Biology (Moscow, 1998).
D. K. Krupatkina, D. A. Selivanovskii, and P. A. Stunzhas, On the buoyancy regulated by means of gas bubbles of one of the colonial forms of the diatom Thalassiosira, Izv. RAN. Ser. Biol., No. 3, 453–461 (1994).
A. S. Lelekov and R. P. Trenkenshu, “Simulation of the dynamics of nitrogenous compounds in microalgae cells. 2. Chemostat,” Mat. Biol. Bioinf. 14 (2), 450-463 (2019).
L. V. Stel’makh and I. M. Mansurova, “Physiological mechanism of survival of dinophyte algae under conditions of biogenic limitation,” Biol. Vnutr. Vod, No. 2, 198–207 (2021).
P. A. Stunzhas and F. V. Sapozhnikov, “These amazing diatoms,” Priroda, No. 5, 12–18 (2000).
M. V. Flint, E. G. Arashkevich, V. A. Artem’ev, et al., Ecosystems of the Seas of the Siberian Arctic. Materials of Expeditionary Research in 2015 and 2017 (Inst. Okeanol. im P.P. Shirshova RAN, Moscow, 2018) [in Russian].
N. Yu. Shoman, Candidate’s Dissertation in Biology (Sevastopol, 2020).
Ecosystems of the Kara Sea and the Laptev Sea. Expedition Research 2016 and 2018 (Izd. Erkhova I.M., Moscow, 2021).
V. I. Belyaev, “Modelling the influence of turbulence on phytoplankton photosynthesis,” Ecol. Model. 60 (1), 11–29 (1992).
A. B. Demidov, S. A. Mosharov, and P. N. Makkaveev, “Patterns of the Kara Sea primary production in autumn: Biotic and abiotic forcing of subsurface layer,” J. Mar. Syst. 132, 130–149 (2014).
A. B. Demidov, V. I. Gagarin, O. V. Vorobieva, et al., “Spatial and vertical variability of primary production in the Kara Sea in July and August 2016: The influence of the river plume and subsurface chlorophyll maxima,” Polar Biol. 41 (3), 563–578 (2018).
K. F. Edwards, M. K. Thomas, C. A. Klausmeier, and E. Litchman, “Allometric scaling and taxonomic variation in nutrient utilization traits and maximum growth rate of phytoplankton,” Limnol. Oceanogr. 57 (2), 554–566 (2012).
J. C. Goldman and J. J. McCarthy, “Steady state growth and ammonium uptake of a fast-growing marine diatom,” Limnol. Oceanogr. 23 (4), 695–703 (1978).
A. G. Kuznetsov, I. V. Konyukhov, S. I. Pogosyan, et al., “Microfluorimeter for studying the state of photosynthetic apparatus of individual cells of microalgae,” Oceanology 61, 1055–1063 (2021).
E. Litchman, C. A. Klausmeier, J. R. Miller, et al., “Multi-nutrient, multi-group model of present and future oceanic phytoplankton communities,” Biogeosciences, No. 3, 585–606 (2006).
M. W. Lomas, S. E. Baer, S. Acton, and J. W. Krause, “Pumped up by the cold: Elemental quotas and stoichiometry of cold-water,” Diatoms. Front. Mar. Sci. 6, 286 (2019).
S. Menden-Deuer and E. J. Lessard, “Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton,” Limnol. Oceanogr. 45 (3), 569–579 (2000).
D. L. Muggli, M. Lecourt, and P. J. Harrison, “Effects of iron and nitrogen source on the sinking rate, physiology and metal composition of an oceanic diatom from the subarctic Pacific,” Mar. Ecol. Prog. Ser. 132 (1/3), 215–227 (1996).
NetLogo. http://ccl.northwestern.edu/netlogo/.
J. S. Parslow, P. J. Harrison, and P. A. Thompson, “Saturated uptake kinetics: Transient response of the marine diatom Thalassiosira pseudonanato ammonium, nitrate, silicate or phosphate starvation,” Mar. Biol. 83 (1), 51–59 (1984).
G.-Y. Rhee and I. J. Gotham, “The effect of environmental factors on phytoplankton growth: Temperature and the interactions of temperature with nutrient limitation,” Limnol. Oceanogr., No. 4, 635–648 (1981).
R. F. Strzepek and N. M. Price, “Influence of irradiance and temperature on the iron content of the marine diatom Thalassiosira weissflogii (Bacillariophyceae),” Mar. Ecol. Prog. Ser. 206, 107–117 (2000).
V. I. Vedernikov, A. B. Demidov, and A. I. Sud’bin, “Primary productionand chlorophyll in the Kara Sea in September 1993,” Oceanology 34, 630–640 (1995).
Funding
This study was performed as part of the state task of Moscow State University (topic no. 121032500060-0) and by the Development Program of the Interdisciplinary Scientific and Educational School of the Moscow State University “The Future of the Planet and Global Environmental Change.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by D. Zabolotny
Rights and permissions
About this article
Cite this article
Fursova, P.V., Riznichenko, G.Y., Konyukhov, I.V. et al. Agent-Based Model of Diatom Population Dynamics in Marginal Arctic Seas in Summer. Oceanology 63, 674–682 (2023). https://doi.org/10.1134/S0001437023050041
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0001437023050041