Abstract
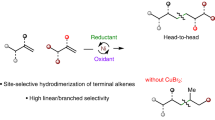
The properties of polymeric materials can be modulated by factors such as sequence control or functional group modifications. However, the synthesis of new macromolecular scaffolds is limited by the accessibility of structurally diverse monomers. This work describes a one-step, nickel-catalysed synthesis of 5,6-diaryl cyclooctene monomers from the feedstock chemical 1,5-cyclooctadiene. The reaction proceeds in a modular, regio- and diastereoselective fashion, granting access to both homo- and hetero-diaryl cyclooctene monomers that smoothly undergo ring-opening metathesis polymerization (ROMP). The resulting 1,2-diaryl-substituted polymers possess sequences with head-to-head styrene dyads that have not been previously explored, giving rise to unique and tunable properties. Density functional theory calculations highlight mechanistic aspects of the nickel-catalysed diarylation reaction and the ruthenium-catalysed ROMP process, revealing a previously unappreciated role of the boronic ester in promoting migratory insertion, which was leveraged to provide enantioinduction.





Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information files. Experimental procedures, characterization data for all new compounds and details for DFT calculations can be found in the Supplementary Information. Crystallographic data for the structure reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 2294340 (2i); copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Ouchi, M., Badi, N., Lutz, J.-F. & Sawamoto, M. Single-chain technology using discrete synthetic macromolecules. Nat. Chem. 3, 917–924 (2011).
Lutz, J.-F., Ouchi, M., Liu, D. R. & Sawamoto, M. Sequence-controlled polymers. Science 341, 1238149 (2013).
Rylski, A. K. et al. Polymeric multimaterials by photochemical patterning of crystallinity. Science 378, 211–215 (2022).
Galdi, N., Buonerba, A. & Oliva, L. Olefin–styrene copolymers. Polymers 8, 405 (2016).
Luo, Y., Baldamus, J. & Hou, Z. Scandium half-metallocene-catalyzed syndiospecific styrene polymerization and styrene–ethylene copolymerization: unprecedented incorporation of syndiotactic styrene–styrene sequences in styrene–ethylene copolymers. J. Am. Chem. Soc. 126, 13910–13911 (2004).
Klosin, J., Fontaine, P. P. & Figueroa, R. Development of group IV molecular catalysts for high temperature ethylene-α-olefin copolymerization reactions. Acc. Chem. Res. 48, 2004–2016 (2015).
McKnight, A. L. & Waymouth, R. M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 98, 2587–2598 (1998).
Derosa, J., Tran, V. T., van der Puyl, V. A. & Engle, K. M. Carbon–carbon π-bonds as conjunctive reagents in cross-coupling. Aldrichimica Acta 51, 21–32 (2018).
Wickham, L. M. & Giri, R. Transition metal (Ni, Cu, Pd)-catalyzed alkene dicarbofunctionalization reactions. Acc. Chem. Res. 54, 3415–3437 (2021).
Qi, X. & Diao, T. Nickel-catalyzed dicarbofunctionalization of alkenes. ACS Catal. 10, 8542–8556 (2020).
Kang, T., Apolinar, O. & Engle, K. M. Ni- and Pd-catalyzed enantioselective 1,2-dicarbofunctionalization of alkenes. Synthesis 56, 1–15 (2024).
Xi, Y. et al. Catalytic asymmetric diarylation of internal acyclic styrenes and enamides. J. Am. Chem. Soc. 144, 8389–8398 (2022).
Derosa, J. et al. Nickel-catalyzed 1,2-diarylation of simple alkenyl amides. J. Am. Chem. Soc. 140, 17878–17883 (2018).
Derosa, J. et al. Nickel-catalyzed 1,2-diarylation of alkenyl carboxylates: a gateway to 1,2,3-trifunctionalized building blocks. Angew. Chem. Int. Ed. Engl. 59, 1201–1205 (2020).
Apolinar, O. et al. Sulfonamide directivity enables Ni-catalyzed 1,2-diarylation of diverse alkenyl amines. ACS Catal. 10, 14234–14239 (2020).
Apolinar, O. et al. Three-component asymmetric Ni-catalyzed 1,2-dicarbofunctionalization of unactivated alkenes via stereoselective migratory insertion. J. Am. Chem. Soc. 144, 19337–19343 (2022).
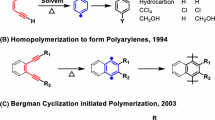
Martinez, H., Ren, N., Matta, M. E. & Hillmyer, M. A. Ring-opening metathesis polymerization of 8-membered cyclic olefins. Polym. Chem. 5, 3507–3532 (2014).
Sutthasupa, S., Shiotsuki, M. & Sanda, F. Recent advances in ring-opening metathesis polymerization, and application to synthesis of functional materials. Polym. J. 42, 905–915 (2010).
Cho, I., Jeong, S. W. & Hwang, K. M. Synthesis and ring-opening metathesis polymerization of substituted cyclooctenes: butadiene-based sequence-controlled copolymers. Korea Polym. J. 1, 1–8 (1993). https://www.cheric.org/PDF/KPJ/KP01/KP01-1-0001.pdf.
You, W. et al. Expeditious synthesis of aromatic-free piperidinium-functionalized polyethylene as alkaline anion exchange membranes. Chem. Sci. 12, 3898–3910 (2021).
Bielawski, C. W. & Grubbs, R. H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 32, 1–29 (2007).
Sathe, D. et al. Olefin metathesis-based chemically recyclable polymers enabled by fused-ring monomers. Nat. Chem. 13, 743–750 (2021).
Kobayashi, S., Pitet, L. M. & Hillmyer, M. A. Regio- and stereoselective ring-opening metathesis polymerization of 3-substituted cyclooctenes. J. Am. Chem. Soc. 133, 5794–5797 (2011).
Zhang, J., Matta, M. E. & Hillmyer, M. A. Synthesis of sequence-specific vinyl copolymers by regioselective ROMP of multiply substituted cyclooctenes. ACS Macro Lett. 1, 1383–1387 (2012).
Katzbaer, J. N., Torres, V. M., Elacqua, E. & Giri, R. Nickel-catalyzed alkene difunctionalization as a method for polymerization. J. Am. Chem. Soc. 145, 14196–14201 (2023).
Han, F.-S. Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: a remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 42, 5270–5298 (2013).
Bhakta, S. & Ghosh, T. Emerging nickel catalysis in Heck reactions: recent developments. Adv. Synth. Catal. 362, 5257–5274 (2020).
Shu, W. et al. Ni-catalyzed reductive dicarbofunctionalization of nonactivated alkenes: scope and mechanistic insights. J. Am. Chem. Soc. 141, 13812–13821 (2019).
Miura, W., Hirano, K. & Miura, M. Nickel-catalyzed directed C6-selective C–H alkylation of 2-pyridones with dienes and activated alkenes. J. Org. Chem. 82, 5337–5344 (2017).
Huang, L. et al. Cascade cross-coupling of dienes: photoredox and nickel dual catalysis. Angew. Chem. Int. Ed. Engl. 59, 457–464 (2020).
Henry, P. M., Davies, M., Ferguson, G., Phillips, S. & Restivo, R. Conversion of cyclo-octa-1,5-diene into 2,6-diacetoxybicyclo[3,3,0]octane by palladium(II) chloride–lead tetra-acetate in acetic acid. X-ray determination of the structure of the product. J. Chem. Soc., Chem. Commun. 112–113 (1974).
Li, Q. et al. Through-space charge-transfer polynorbornenes with fixed and controllable spatial alignment of donor and acceptor for high-efficiency blue thermally activated delayed fluorescence. Angew. Chem. Int. Ed. Engl. 59, 20174–20182 (2020).
Cui, J., Yang, J.-X., Pan, L. & Li, Y.-S. Synthesis of novel cyclic olefin polymer with high glass transition temperature via ring-opening metathesis polymerization. Macromol. Chem. Phys. 217, 2708–2716 (2016).
Tsuji, J. & Takahashi, H. Organic syntheses by means of noble metal compounds. XII. Reaction of the cyclooctadiene-palladium chloride complex with ethyl malonate. J. Am. Chem. Soc. 87, 3275–3276 (1965).
Krafft, M. E., Sugiura, M. & Abboud, K. A. Novel use of ring strain to control regioselectivity: alkene-directed, palladium-catalyzed allylation. J. Am. Chem. Soc. 123, 9174–9175 (2001).
Ney, J. E. & Wolfe, J. P. Synthesis and reactivity of azapalladacyclobutanes. J. Am. Chem. Soc. 128, 15415–15422 (2006).
Gandeepan, P. & Cheng, C.-H. Allylic carbon–carbon double bond directed Pd-catalyzed oxidative ortho-olefination of arenes. J. Am. Chem. Soc. 134, 5738–5741 (2012).
Qiu, Y., Yang, B., Zhu, C. & Bäckvall, J.-E. Highly selective olefin-assisted palladium-catalyzed oxidative carbocyclization via remote olefin insertion. Chem. Sci. 8, 616–620 (2017).
Zheng, X.-W. et al. Mechanistic insight into the selective olefin-directed oxidative carbocyclization and borylation by a palladium catalyst: a theoretical study. RSC Adv. 7, 5013–5018 (2017).
Johnson, J. B. & Rovis, T. More than bystanders: the effect of olefins on transition-metal-catalyzed cross-coupling reactions. Angew. Chem. Int. Ed. Engl. 47, 840–871 (2008).
Tran, V. T. et al. Ni(COD)(DQ): an air-stable 18-electron nickel(0)–olefin precatalyst. Angew. Chem. Int. Ed. Engl. 59, 7409–7413 (2020).
Tran, V. T. et al. Structurally diverse bench-stable nickel(0) pre-catalysts: a practical toolkit for in situ ligation protocols. Angew. Chem. Int. Ed. Engl. 62, e202211794 (2023).
Gao, P., Chen, L.-A. & Brown, M. K. Nickel-catalyzed stereoselective diarylation of alkenylarenes. J. Am. Chem. Soc. 140, 10653–10657 (2018).
Estrada, J. G., Williams, W. L., Ting, S. I. & Doyle, A. G. Role of electron-deficient olefin ligands in a Ni-catalyzed aziridine cross-coupling to generate quaternary carbons. J. Am. Chem. Soc. 142, 8928–8937 (2020).
Allinger, N. L. & Sprague, J. T. Conformational analysis. LXXXIV. A study of the structures and energies of some alkenes and cycloalkenes by the force field method. J. Am. Chem. Soc. 94, 5734–5747 (1972).
Sanford, M. S., Love, J. A. & Grubbs, R. H. A versatile precursor for the synthesis of new ruthenium olefin metathesis catalysts. Organometallics 20, 5314–5318 (2001).
Martinez, H., Miró, P., Charbonneau, P., Hillmyer, M. A. & Cramer, C. J. Selectivity in ring-opening metathesis polymerization of Z-cyclooctenes catalyzed by a second-generation Grubbs catalyst. ACS Catal. 2, 2547–2556 (2012).
Nowalk, J. A. et al. Sequence-controlled polymers through entropy-driven ring-opening metathesis polymerization: theory, molecular weight control, and monomer design. J. Am. Chem. Soc. 141, 5741–5752 (2019).
Liu, B., Wang, L., Wu, C. & Cui, D. Sequence-controlled ethylene/styrene copolymerization catalyzed by scandium complexes. Polym. Chem. 10, 235–243 (2019).
Acknowledgements
Financial support for the experimental work was provided by the Department of Energy (DE-SC0023205). The computational work was supported by the National Science Foundation (CHE-2102550). We thank the Schimmel Family Endowed Scholarship Fund for a Graduate Fellowship (C.Z.R.). We thank the Independent Research Fund Denmark (grant ID 10.46540/3102-00009B) for financial support (A.K.R.). Computational studies were performed at the Center for Research Computing at the University of Pittsburgh and the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) program supported by NSF. We acknowledge Dr. M. Gembicky (UCSD) for X-ray crystallographic analysis.
Author information
Authors and Affiliations
Contributions
V.T.T., A.K.R. and C.Z.R. performed optimization and evaluated the scope of the nickel-catalysed 1,2-dicarbofunctionalization reaction. M.X. and E.M.W. carried out polymerization experiments. Y.F. and P.L. carried out computation work. S.R.W. performed kinetic experiments on the nickel-catalysed 1,2-dicarbofunctionalization reaction. V.T.T., P.L., W.R.G. and K.M.E. conceived the project. A.K.R., P.L., W.R.G. and K.M.E. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Yifeng Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental procedures, characterization data for all new compounds and details for DFT calculations. Supplementary Figs. 1–132 and Tables 1–16.
Supplementary Data 1
Crystallographic data for 2i, CCDC 2294340.
Supplementary Data 2
Raw NMR spectroscopic data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tran, V.T., Ravn, A.K., Rubel, C.Z. et al. Ni-catalysed dicarbofunctionalization for the synthesis of sequence-encoded cyclooctene monomers. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00618-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00618-1
- Springer Nature Limited
This article is cited by
-
Diarylation encodes precision for polyolefins
Nature Synthesis (2024)