Abstract
Vegetated coastal ecosystems (mangroves, seagrasses, and saltmarshes, often called Blue Carbon ecosystems) store large carbon stocks. However, their regional carbon inventories, sequestration rates, and potential as natural climate change mitigation strategies are poorly constrained. Here, we systematically review organic carbon storage and accumulation rates in vegetated coastal ecosystems across the Central and Southwestern Atlantic, extending from Guyana (08.28°N) to Argentina (55.14°S). We estimate that 0.4 Pg organic carbon is stored in the region, which is approximately 2-5% of global carbon stores in coastal vegetated systems, and that they accumulate 0.5 to 3.9 Tg carbon annually. By ecosystem type, mangroves have the largest areal extent and contribute 70-80% of annual organic carbon accumulation, with Brazil hosting roughly 95% of mangrove stocks. Our findings suggest that organic carbon accumulation in the region is equivalent to 0.7 to 13% of global rates in vegetated coastal ecosystems, indicating the importance of conserving these ecosystems as a nature-based approach for mitigating and adapting to climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Blue Carbon (BC) designates the carbon dioxide (CO2) captured from the atmosphere and stored by coastal and marine ecosystems1. The BC concept emphasizes the importance of conservation and restoration of those vegetated coastal ecosystems (VCE) to ensure their contribution to climate mitigation and adaption1,2,3. As VCE are undergoing conversion and/or degradation at increasing rates (up to 3% per year)4, they may switch from carbon sinks into CO2 sources, adding to anthropogenic greenhouse gas (GHG) emissions, summing those caused by terrestrial deforestation, land-use change and the burning of fossil fuels5. BC needs to be accountable and amenable to management in order to be used for climate mitigation strategies and as conservation tools6.
Given their efficiency on a per area basis as organic carbon (OC) sinks over timescales from decades to even millennia, the VCE of mangroves, saltmarshes, and seagrass meadows are major components of BC. Albeit occupying only ~0.5% of the seafloor, the VCE contribute to ~50% of worldwide OC burial in marine soils, exceeding those of terrestrial forests, per unit of area, by 1–2 orders of magnitude3. Global interest in VCE is thus rooted in their potential to provide natural climate solutions (NCS) to achieve the target of the Paris Agreement of limiting global warming while generating a range of other essential ecosystem services such as nutrient removal7, fisheries, and biodiversity enhancement8. They also provide protection against the impacts of sea-level rise and extreme climatic events, increasing coastal resilience, protecting local communities, safeguarding food security, and reducing the costs of adaptation4. VCE are thus of extreme value to countries, especially those with moderate fossil fuel emissions and extensive VCE along their coastlines6.
Geomorphology, hydrology, and climate are essential components determining macroscale variation in VCE processes9. As a result, each VCE has a set of environmental conditions that control the type and rate of sediment supply, origin of organic carbon (autochthonous vs. allochthonous), organic matter diagenesis, and C storage in vegetation and soil pools10,11. Thus, reconciling regional and macroscale data in VCE is vital in advancing conceptual and predictive carbon-climate interactions.
Robust regional assessments of OC stocks and accumulation rates in VCE are needed in order to provide a consistent global budget and to inform policymakers of the regional and national potential for developing conservation and climate change policies and requirements and supporting the inclusion of VCE in Nationally Determined Contributions (NDC)12. As VCE fall within the definition of ‘coastal wetlands’ and mangroves can be classified as ‘forests’, they are eligible for inclusion within national greenhouse gas inventories according to the guidance provided by the Intergovernmental Panel on Climate Change13.
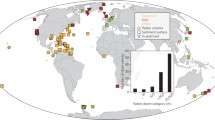
Currently available BC data reflects different regional research efforts and capabilities and is geographically biased. The evidence for known or suspected hotspots of BC, such as along the Southwestern Atlantic (SWA) coast, is a large knowledge gap (Fig. 1). The patchy information available on regional and national C inventories and sequestration rates has severely limited the understanding of the contribution of VCE to climate change mitigation and the implementation of national BC strategies in the SWA. This contrasts to the nearby Amazonia Forest region where robust estimates are available and losses or gains in the forest area are known in quasi-real time14. There is, therefore, an urgent need to identify and address current knowledge gaps to respond to conservation, policy, and management requirements and opportunities.
Spatial distributions of VCE in green. Location of the study area (A) and sites from where data from sediment cores (B, C, D; orange triangles) and aboveground biomass (E, F, G; yellow circles) were extracted. GU Guianan, AM Amazonia, NB Northeastern Brazil, FN Fernando de Noronha and Atol das Rocas, EB Eastern Brazil, SB Southeastern Brazil, RG Rio Grande, RP Rio de la Plata, UB Uruguay–Buenos Aires Shelf, NP North Patagonian Gulfs, PS Patagonian Shelf, and FM Malvinas/Falklands.
Here we systematically review, compile, and synthesize the available data on aboveground biomass and soil (belowground biomass + soil) OC stocks, as well as coastal OC sequestration rates for the SWA from Guyana (08.28°N) to Argentina (55.14°S). The region holds potential BC hotspots, including the world’s highest concentration of mangroves along the equator15. The SWA also has extensive subtropical and temperate saltmarshes16 and seagrass meadows17 that remain undervalued in global estimates. In doing so, we consolidate the available knowledge, identify data gaps and propose directions for future steps to improve the BC research and agenda for the region. We also estimate the potential contribution of BC inventories to support the conservation of these ecosystems and to implement NDC, including adaptive capacity to address climate-related hazards.
Results and Discussion
Distribution of VCE and knowledge gaps across the SWA
The region (Fig. 1) holds one of the largest global areas of VCE, which is estimated at ~1.5 Mha (Table 1). Regional assessments of mangrove forests cover are more consistent than saltmarshes or seagrass meadows due to the high availability and cover of satellite images, the ability to discriminate between tree species, and the general lack of boundary ambiguities18,19. Mangroves have the greatest areal extent of VCE in SWA (1.2 Mha), representing 9–15% of the global mangrove areal extent (Table 2), with most mangrove forests in Brazil (1.05 Mha; Supplementary Table S1). Our estimate of the mangrove area is commensurate with other recent estimates for the SWA region (1.2–1.4 Mha20; 1.5 Mha21; 1.2 Mha22). There are 3 genera of mangrove trees in the region, with a total of six mangrove species: the red mangrove (Rhizophora mangle, Rhizophora harrisonii, and Rhizophora racemosa), the white mangrove (Laguncularia racemosa) and the black mangrove (Avicennia schaueriana and Avicennia germinans)23.
Despite the scattered distribution, seagrass meadows occur along most of the SWA coast (Fig. 1). Seventeen species of seagrasses are reported for the Tropical Atlantic and Caribbean24, but only five occur along the SWA (i.e., Halophila baillonii Ascherson, Halophila decipiens Ostenfeld (Hydrocharitaceae), Halodule wrightii Ascherson, Halodule emarginata Hartog (Cymodoceaceae) and Ruppia maritima Linnaeus (Ruppiaceae)17. Among them, Ruppia maritima has the widest latitudinal distribution but is confined to shallow estuarine areas whereas Halodule wrightii is most abundant overall17.
Estimates of global seagrass distribution differ greatly in the literature (from 1625 to 60 Mha26), with models suggesting five times more seagrass habitats than the mapped global distribution25. Our estimate (0.073 Mha) is equivalent to ~0.1–0.4% of the mapped global area (Table 2) and the percentage would be two orders of magnitude lower if we considered the maximum global extent estimated via modeling27. However, the model generates inconsistent results compared to in situ observations, predicting extensive seagrass meadows along the muddy coast of the Amazon River delta and adjacent continental shelf, where the high turbidity and sediment instability are not suitable for seagrass growth and seagrasses have not been reported17. Our estimation did not account for the deep-water seagrass Halophila decipiens, which is known to occur down to 60 m in clear oligotrophic waters of Eastern Brazil and probably covers significant areas on the still poorly studied South Atlantic continental shelf. Mapping seagrass meadows remains challenging due to the dominance of small-leaved species that form patchy meadows in turbid coastal waters or deep clearer waters. The global modeling parameters may not be appropriate and accurate estimates of the areal extent of seagrass distribution remain a major challenge. Given the current limitations our assessment of the areal extent of seagrass in SWA is therefore conservative.
Saltmarshes in the SWA (0.18 Mha) represent ~4–8% of global areal extent (Table 2). Although our regional saltmarsh areal estimates are similar to those in Macreadie et al.20 (0.13 Mha), on a national basis their distribution differs considerably and better mapping is required to arrive at a consensus. The largest areas of saltmarsh in SWA are concentrated in the subtropical and temperate regions (between 29° and 54° S, in South Brazil, Uruguay and Argentina, Supplementary Table S1), where completed mapping and species habitats coverages are both available (ref. 16 and references therein). Further north (29° S to the equator), saltmarshes occur in smaller areas, mainly associated with the more dominant mangrove forests. In this situation, the distinction between the boundaries of these two VCE is mostly ill-defined in mapping studies or is not distinguished from contiguous freshwater marshes. The floristic diversity found in saltmarshes shows latitudinal variation, with lower diversity near the Equator (five species) and higher variety in the temperate zone (twenty species)28. The three main species that dominate the SWA’s low and middle intertidal saltmarshes are Spartina alterniflora, Spartina densiflora, and Sarcocornia perennis29.
We identified important data gaps in mangrove biomass along parts of the coastline of the Guianan, Amazonia and Northeastern Brazil ecoregions (Fig. 1). Mangrove soil data was also patchy across ecoregions, with very little information from the Guianan and limited data along most of the coastline of the Amazonia, half of Northeastern Brazil and one third of the Eastern Brazil (Fig. 1). Relatively more data was available on seagrass biomass and it was better distributed throughout the known geographical extension of these habitats. Data for seagrass soil OC was contrastingly rare and highly localized (i.e., Northeastern Brazil, Southeastern Brazil and Rio Grande marine ecoregions; Fig. 1). The largest seagrass soil data gaps were in Northeastern and Eastern Brazil, where seagrass meadows thrive along tropical reefs and within estuaries. For saltmarshes, biomass measurements were also well distributed throughout their known geographical distribution16,29 but no data was available on saltmarsh soils in the Guianan, Northeastern and Eastern Brazil marine ecoregions (where tropical saltmarshes occur associated with mangroves) or in the Patagonian Shelf (dominated by extensive saltmarshes).
Improved mapping of the SWA ecosystems, particularly for seagrasses and saltmarshes, is required to assess the contributions of these ecosystems to national and regional CO2 sinks and stores and their importance for climate change adaptation/mitigation. As a result, the estimates presented here are conservative, likely at the lower value range limits, but it is the first step towards more refined quantification of OC stores and the adoption of strategies for climate mitigation .
C stocks across the SWA
The total OC stock (aboveground biomass + the top 1 m belowground components—biomass and soil) of VCE was estimated as holding 404 Tg C, with most of the C (72%) contained in the soils (Fig. 2 and Table 1). The relative contribution of aboveground biomass from each VCE to the total ecosystem C stocks is similar to those recently reported for Australian VCE30.
Mangroves accounted for 83% (337 Tg C) of the total OC stocks, with their soil representing about 67% of the total stock. The higher C storage in mangroves soils compared to either seagrasses or saltmarshes is consistent with other studies4,30,31. In saltmarshes and seagrass meadows, soil stocks accounted for ~99% of their total stocks and these VCE accounted for 14% (55 Tg C) and 3% (12 Tg C), respectively, of the total stocks in the SWA and 19% and 4%, respectively, of the soil stocks in the SWA. From a national perspective, saltmarshes accounted for ~100% of OC stocks in Argentina and Uruguay, while mangroves represented 93% of the Brazilian OC stocks (Table S1). Nearly 100% of seagrass OC stocks of the SWA were found in Brazil. In terms of aboveground and soils OC stocks per unit area, the average values were significantly different between the VCEs (Fig. 2, Supplementary Table S2), whereas considering the sum of aboveground and soil stocks, mangroves and saltmarshes stocks are comparable (330 and 380 Mg C ha−1, respectively; Table 1).
The mean aboveground mangrove OC stock per unit area (72 Mg C ha−1) was 225-fold and 9-fold higher than for seagrass (0.32 Mg C ha−1) and saltmarsh (7.8 Mg C ha−1), respectively. For soils there were also significant differences (P < 0.05, Supplementary Table S2) but the mean OC stock per unit area was highest for saltmarshes (372 ± 47 Mg C ha−1), followed by mangroves (259 ± 151 Mg C ha−1) and seagrass (91 ± 82 Mg C ha−1). Comparing stocks in the SWA with global averages, mangroves stocks are within global ranges (283–369 Mg C ha−1)32,33. The calculated mean and standard deviation of OC stocks in saltmarsh soils are strongly influenced by the high OC densities reported for the Falklands/Malvinas, resulting in a value greater than the global average20. Falkland Island saltmarshes are known to have extensive coverage of one of the highest carbon-dense peats34 in the world, and a contiguous distribution with freshwater and grass peatlands may result in ill-defined boundaries between saltmarshes, freshwater marshes, and upper grasslands. In this review, the only soil cores used34 were extracted from well-developed saltmarshes in the Falkland Islands. Excluding the data from this region, the resulting saltmarsh average OC stock (270 ± 105 Mg C ha−1) would be comparable to global estimates. Besides, this value is equivalent to the C storage estimated (270 ± 130 Mg C ha−1) for the conterminous US coastal wetlands (~2000 cores from estuarine wetlands, tidal palustrine, estuarine and tidal palustrine forest and scrub) top 1 m of soil.
In contrast the OC stocks for seagrass soils in the SWA (91 ± 82 Mg C ha−1) are in the lower range of global estimates (140 Mg C ha−1)31, likely reflecting the low density and patchy seagrass beds composed by smaller, more ephemeral species17, when compared to the persistent species and dense seagrass meadows of the Mediterranean, Caribbean and Australasian regions.
There was large variability in total ecosystem OC stocks across ecoregions (Fig. 3, Supplementary Table S3), ranging from 20 to 244 Tg C in mangroves, 0.2 to 31 Tg C in saltmarshes and 0.003 to 11 Tg C in seagrasses. A large part of the spatial variability observed can be explained by the areal extent of the VCE (Fig. 1). There was also substantial variability in OC stocks per unit area across the ecoregions in SWA (Fig. 3, Supplementary Table S4). The mangroves in Amazonia contain ~3-fold higher aboveground stocks per unit of area (132 ± 48 Mg C ha−1) than the subtropical Southeastern Brazil ecoregion (41 ± 24 Mg C ha−1; P = 0.0003; Supplementary Table S5). There is a decreasing trend in the mangrove soil stocks per unit area from the subtropical to the equatorial SWA, with those from the Southeastern Brazil ecoregion (303 ± 196 Mg C ha−1) being ~4 and 2-fold higher than in Guiana and Amazonia, respectively. Mangrove soils (Supplementary Table S3) in Guianan and Amazonia ecoregions have lower OC contents (2.7 ± 4.4% and 2.6 ± 0.64%, respectively) than other ecoregions (averages of 4.3 ± 5.4% in Eastern Brazil to 7.13 ± 6.85% in Southern Brazil). The low OC content in soils of the equatorial region is associated with soil pedogenesis and hydrodynamic conditions. The large tidal range in the northern ecoregions decreases the residence time of organic matter and reduces the period that soils experience anoxic conditions35. In addition, the predominant coarse-grained soils36 in this area do not promote the physical and chemical protection provided by fine-grained soils37. Differences in the observed soil OC contents between Amazonia and Guianan compared to other ecoregions may also reflect the strong influence of freshwater discharge and its accompanying particulate load that is advected to the south of the Amazonian delta38. The use of stable C isotopes and other biomarkers (e.g., eDNA, fatty acids) to investigate the sources of soil organic matter could allow the drivers of the variability of C stocks to be discerned across the SWA.
For seagrasses, the lowest aboveground biomass (0.14 ± 0.21 Mg C ha−1) and soil (51 Mg C ha−1) OC stocks were found in the subtropical region of the Southeastern Brazil and the Rio Grande ecoregions, respectively, whereas in the tropical Northeastern Brazil stocks are ~4-times higher (aboveground 0.59 ± 0.71 Mg C ha−1 and soil 214 ± 129 Mg C ha−1). The regional difference in seagrass stocks (Supplementary Table S6) may be related to a complex interaction among climate, pedogenesis, geomorphology and seagrass meadow characteristics. Along the semiarid coast in the Northeastern ecoregion the tropical Halodule species form dense, stable meadows with high biomass adjacent or contiguous to mangrove forest within estuaries17. In Southeastern Brazil, seagrasses occur in marine carbonate soils, forming low density and more ephemeral meadows39. Further south in the subtropical humid Rio Grande ecoregion, seagrass meadows are dominated by the mostly ephemeral and eurihaline Ruppia maritima that forms annual meadows together with other oligohaline and freshwater grasses in low OC soils in coastal lagoons17. Studies in other regions have shown that at the continental scale the magnitude of OC deposits in seagrass soils did not differ between regions or habitat geomorphic settings, but was higher in meadows dominated by large compared to those dominated by small species40. The environmental settings of the meadow, including the origin of OC (authochtonous vs. allochthonous) and the presence of anthropogenic stressors, have also been considered important factors controlling OC stocks40,41,42.
OC stocks per unit area were lowest in Amazonia saltmarshes compared to those in all other ecoregions (1.5 ± 1.2 Mg C ha−1 and 72 ± 12 Mg C ha−1, above and belowground, respectively; Fig. 3), although not all differences for aboveground stocks were significant (Supplementary Table S7). Across the subtropical-temperate ecoregions, saltmarsh soil stocks per unit area were of the same magnitude, except for the Falklands/Malvinas ecoregion that was up to 5-fold higher (1086 ± 138 Mg C ha−1). Between and within ecoregions site-specific properties such as granulometry, nutrient contents of soils, plant species, geomorphology, level of soil inundation, precipitation and tidal regimes may all explain the observed distribution patterns in saltmarsh stock across the ecoregions43,44.
Trends and gaps in C accumulation rates
Mangroves have been the focus of research on VCE in the SWA and there are several studies reporting estimates of OC accumulation rates (CAR, 18 cores) or mass accumulation rates (MAR, 33 cores) from which CAR may be estimated (Supplementary Table S3). In contrast, there is no published data for soil accumulation rates in saltmarshes and only 2 estimates of CAR (0.11 and 0.34 Mg C ha−1 yr−1) for seagrass, both derived from Ruppia maritima meadows in the Patos lagoon, South Brazil45. Nine additional seagrass (Halodule wrightii) soil cores recently sampled in the Todos os Santos Bay, Eastern Brazil have showed the lack of net accumulation or a very limited accumulation of fine particles at a rate that could not be determined, but suggesting very low CAR (Hatje et al. unpublished data).
Given the available data, our best estimate of the total annual sequestration rates for VCE in SWA ranges from 0.5 to 3.9 Tg C yr−1 (Table 2), with mangroves contributing between 70–80% of annual sequestration. Although this is the most comprehensive compilation of available data to date, there are substantial gaps in our knowledge of CAR. This will potentially cause errors to the regional estimate but also highlights the need to obtain more data. Thus, the total annual sequestration rates estimated here are poorly constrained and most likely underestimated. Future research is necessary to further refine our estimates.
Notwithstanding the limited data, the annual sequestration rates in mangrove soils were up to 2 orders of magnitude higher than in seagrasses and twice the maximum rates in saltmarshes (Table 2). The CAR (Supplementary Table S3) for mangroves varied over two orders of magnitude across the SWA (0.1−11 Mg C ha−1 yr−1), with a mean of 2.6 ± 1.9 Mg C ha−1 yr−1, (median of 2.9 Mg C ha−1 yr−1), which is higher than the global average of 1.6 ± 0.4 Mg C ha−1 yr−1 46 and the average estimated in Australia (1.3 ± 0.9 Mg C ha−1 yr−1)30. There is a decreasing trend in the mean CAR per unit of area and total sequestration rates in mangroves from Southeastern Brazil to the Amazon, and relatively high values in the Guianan ecoregion (Supplementary Table S3). It is known that the soil OC content, in general, accounts for only ~10% of the variation that exists in the C burial rates46. However, for the SWA region the OC contents generally followed a similar trend to burial rates (Supplementary Table S3).
The controlling factors of VCE sequestration rates are an interplay of environmental conditions (e.g., geomorphology, tidal amplitude, climate, proximity to rivers, sediment load), biotic factors (e.g., production rates, turnover time, species composition), and anthropogenic influences37,47,48,49, but globally there is still a scarcity of information on within and between ecosystem variability50,51. Our review demonstrates that there is a dearth of published estimates of CAR in seagrass and saltmarsh habitats in SWA and, while slightly better, the situation is far from ideal for mangroves. Although we identified a latitudinal variation in CAR for mangroves, there is not enough information to test hypotheses regarding the relative importance of riverine OC or tidal fluxes (i.e., potentially important sources of allochthonous carbon) compared to other drivers such as temperature, precipitation, forest structure, and anthropogenic impacts.
Importance and vulnerability of SWA blue C hotspots
Based on the available global data, we estimated that SWA holds between 1 and 4% (0.29 Pg C; Tables 1 and 2) of the global soil OC stocks in VCE, as well as ~1–4% of the global annual OC burial (Table 2)30,31,32,52. However, we provide conservative estimates as there are significant questions regarding: (i) the true distribution and areal extent of saltmarshes and seagrass meadows; (ii) the limited data on OC stocks and sequestration rates; and (iii) that OC stocks assessments are limited to the top 1-m of soil, but taking into account the soil depths reported for some SWA mangroves (up to 3 m)37,50,53,54, saltmarshes (1 to >3 m)53,55, and seagrasses (0.5–2 m)56,57, the C storage for the region is certainly higher.
Despite the different methodologies, our estimate of mangrove stocks for Brazil (318 ± 83 Tg C) is in excellent agreement with the recent Rovai49 estimate (320 ± 70 Tg C). We used the most updated and accurate value for the Brazilian mangrove area, which has been monitored since198558 and is around 30% higher than the value used by Rovai et al.49. In addition, Rovai et al.49 used an aboveground: belowground ratio of 0.5 to estimate belowground mangrove biomass. To the best of our knowledge there is only one study that has evaluated this ratio in SWA, which indeed indicated that belowground fine roots (<5 mm) could represent more than 50% of the C stock in mangrove59. However, most of the studies in mangroves soils do not separate fine roots, that are usually incorporated in soils stocks. This reinforces that limited available data and, possibly, lack of well-established methodologies are major issues, which need to be addressed to assess the potential of VCE in reducing global climate change impacts. If we were to consider the ratio of 0.5 our total estimates for the SWA would increase by 17% to 370 Tg C.
Two percent of Brazilian mangroves were lost between 1999 and 2018, an average rate of ~0.1% per year60, which is in line with global trends (total loss of 2.1%; 0.13% per year, estimated from 2000 to 201622). Annual losses for French Guiana, Guyana and Suriname were estimated at 0.24%, 0.6%, and 0.38%, respectively, for this same period, using Goldberg et al.22 mangrove land-use change. The main drivers of change for SWA mangroves are attributed to shoreline erosion, extreme weather events, clearing, shrimp and salt ponds22,49,61.
The total potential CO2eq emissions from mangrove loss in Brazil is estimated as ~12,000 Mg CO2e ha−1 49. Across Brazil the highest emissions resulting from mangrove forest loss are driven by erosion, particularly at the Amazonia and Northeastern Brazil ecoregions. In Northeastern Brazil, conversion of mangroves to shrimp ponds has resulted in losses of 58–82% of the mangrove carbon stocks of the affected forests, with potential emissions of 1390 Mg CO2e ha−1 62. Future projections (2010–2100) of mangrove loss across the north Brazilian shelf and warm temperate and tropical Southwest Atlantic suggest cumulative emissions of about 143 × 106 Mg CO2e to the atmosphere21. Financially viable projects in mangroves (Brazil, French Guiana, Guyana, and Suriname) of the SWA have been estimated to potentially contribute 931,000 tCO2 for climate mitigation, equivalent to about 0.2% of the annual emissions for the region, with a return of 24.9 million US$ yr−1 (using values reported in Zeng et al.63). Using our data (Table 1), the deforestation rate and methodological approach taken by Atwood et al.32, and assuming that the loss rates for seagrass and saltmarsh are similar to those of mangroves, the current annual emissions for the SWA would equate to 0.7 × 106 Mg CO2e yr−1.
Impacts of global changes are known to be affecting the SWA mangroves61,64, saltmarshes65 and seagrass habitats17,66, although the effects vary according to ecoregion, ecosystem, and the interacting anthropogenic pressures. Most is known about mangroves in Brazil. For example, ecosystem C losses have been reported for Southeastern Brazil following climate-induced mangrove mortality with emissions of ~993 Mg CO2e ha−1 67. Landward migration and land-use change appear to be the main responses of mangroves in Northeastern Brazil to sea-level rise and coastal erosion61, which may alter nutrient cycling in the future. In Southeastern Brazil, the potential for landward migration is limited by the narrow coastal plain as well as dense urbanization (e.g., roads, urban centers). The austral mangrove limit, between the Southern Brazil and Rio Grande ecoregions, has shifted south over the last decades, as mangroves encroach coastal wetlands due to global warming68.
Conclusions
The SWA is a hotspot for VCE holding stocks of OC (0.4 Pg) equivalent to 2–5% of global soil estimates and 0.7 to 4% of annual worldwide sequestration rates. Mangroves are the greatest VCE contributor in terms of total stock (337 Tg C) and sequestration rates (0.4–2.6 Tg C year−1), whereas stocks per unit of area are equivalent in mangroves and saltmarshes. Despite the important knowledge gaps identified (e.g., in seagrass and saltmarshes distribution, data coverage for stocks and accumulation rates), the SWA stands to benefit from NCS and inclusion of VCE in national and regional carbon credit markets. Furthermore, the region is important within the context of global carbon management and policies. The current and historical losses of VCE could be mitigated through conservation and restoration of impacted areas, while enhancing diverse ecosystem services. The conservative estimates provided here are, to the best of our knowledge, the first consolidated effort to: (1) fix a baseline of BC in SWA, reducing the bias in the global dataset on C stocks and accumulation rates; (2) provide an essential underpinning to protect existing VCE; (3) assess future impacts on land-use changes and climate changes on C stocks and fluxes. The magnitude of the VCE in the SWA region indicates that carbon finance could provide funds for marine coastal ecosystem conservation and restoration implementing large-scale efforts to protect VCE in the region.
However, further research is required to fill identified gaps. Geographic biases in the VCE coverage and BC data must be addressed, particularly for seagrasses and saltmarshes. Robust information on the scope of country and regional scale should be developed, including improved estimates of the influence of anthropogenic disturbances and climate change on VCE, thereby providing more meaningful data to implement blue carbon into climate change mitigation strategies for policy.
Methods
General approach for literature searches
We compiled the available data on C stocks in soils and aboveground biomass, C accumulation rates, and metadata (e.g., core length, C contents, loss on ignition, dry bulk density, vegetation biomass, among others) in mangrove, saltmarsh, and seagrass meadows in the Central and Southwestern Atlantic coast.
The studied region extends from Guyana (08.28°N) to the Beagle Channel, Tierra del Fuego (Argentina, 55.14°S), which includes the North Brazil Shelf, Tropical Southwestern Atlantic, Northeastern Brazil, Southeastern Brazil, Warm Temperate Southwestern Atlantic Province, as well as the North Patagonian Gulfs regions of the Magellanic Province, according to the Marine Ecoregions of the World (MEOW)69. The data recovered was heterogeneous and the methods employed by the evaluated studies varied in details. As for data quality, we favored the use of dry bulk density (DBD), C data as concentration, density, stocks, or organic C concentration for individual cores. Only less than 10% of the data had to be converted from Loss on Ignition (LOI).
The literature search included peer-reviewed publications, Ph.D. thesis, and MSc dissertations recovered from the Web of Science, Google Scholar, and Periódicos CAPES (Brazilian national database for dissertations and thesis). Details of the procedures for the literature search are provided in the Supplementary methods.
For mangrove forests, data from 74 study sites on soil C stocks (198 cores), soil accumulation rates (33 cores), and standing C stocks in aboveground biomass (146 plots) were used in this study. For saltmarshes, we compiled data for 167 study sites, which correspond to 293 cores of soil C stocks (biomass + soil) and 227 plots for standing C stocks in aboveground biomass. No data on soil accumulation rates was identified. For seagrasses, data from 248 study sites on soil C stocks (104 cores), soil accumulation rates (2 cores), and standing C stocks in aboveground biomass (275 plots) were compiled.
Estimates of biomass and carbon in biomass and soils
For mangroves, we used aboveground biomass estimates from the literature averaged per site and per ecoregion. A conversion factor of 0.46 was used to estimate C in biomass70. We used mean total values for seagrass biomass (above and belowground) taken from the publications. Where above and belowground values were given, the total biomass was calculated as the sum; when only maximum and minimum values were given, total biomass was estimated as the mean. We used a factor of 0.3531 to convert the biomass of seagrass to C. For saltmarshes, we compiled the above and belowground data. When belowground data was absent, we used specific belowground: aboveground ratios for each species calculated from local and regional data. When specific C data was absent, we used the conversion factor of 0.4145,71.
The values of loss on ignition (LOI) for mangrove soil profiles (~9% of the data) were converted to the percentage of C using the equation proposed by Howard et al.70. We used the pedotransfer function to estimate dry bulk density (DBD) of soils for mangrove studies that did not report one32. This was only performed for a few studies since the pedotransfer function only gives an approximation of the true value. For saltmarshes, the LOI conversions were made using (LOI * 0.47) + 0.000870. When no DBD was available for saltmarshes, it was estimated using DBD (Mg m−3) = (2.684–140.943) * 0.08 * exp [–0.008 x OC (g k – 1)]72. All seagrass core samples used in this study have associated DBD data. LOI conversion was used in only a few seagrass cores by applying empirical equations (0.2301*LOI-0.0068).
Whenever possible individual core records were used and means were calculated from tables or figures. To allow comparison among studies, soil C stocks were estimated down to 1 m by summation of the C content per unit area (C densities * slice thickness) of each depth interval70. If necessary, we estimated C stocks down to 1 m by extrapolating linearly integrated C content values with depth. Specific details for each ecosystem, when necessary, are presented in the Supplementary methods.
We endeavored to ensure that the data gathered was of the highest quality possible, although there are methodological differences and unknown uncertainties in the database (as for any large spatial scale compilation study), which contribute to uncertainties in stocks and burial rate estimates. For instance, not all publications reported quality assurance/quality control procedures, reported whether or not results were corrected for the occurrence of inorganic carbon or if roots were excluded from soils before sample processing.
Upscaling to ecoregions and ecosystems
The upscaling of C stocks for each ecoregion69 was performed by multiplying the areas of each ecosystem (Supplementary Table S3) by the average aboveground biomass and the soil C stocks (Supplementary Table S4). For mangroves, the area coverage per ecoregion for Brazil was obtained from the MapBiomas project (www.mapbiomas.org) in MapBiomas Collection 5.058, and we used the lower bound areas for the Guianan ecoregion20,32. To estimate the area of seagrass meadows a series of steps were taken: (1) we created a database of 196 sites (Supplementary Table S8) for the studies from which we extracted biomass or soil carbon data; (2) we extracted the regions data from UN Environment World Conservation Monitoring Centre Global distribution of seagrasses database73—116 points and 1 polygon after removal of repeated points; (3) we combined the two information sources and removed duplicates yielding a total of 316 points and one polygon; (4) using expert knowledge and based on published studies we assigned each seagrass meadow (point) a 1 km2 area; (5) the total area of each ecoregion was determined as the number of points in the ecoregion × 1 km2 + polygon area in the ecoregion (Supplementary Table S8). Saltmarsh occurrence and coverages were compiled from UNEP-WCMC Global Distribution of Saltmarsh dataset74, complemented with the regional maps and literature (Supplementary Table S9). The SWA tropical ecoregions saltmarsh coverage was estimated by summing most studied areas along the coast (Supplementary Table S9). The area of each ecosystem for the Southwestern Atlantic and the estimates of C stocks and sequestration rates are presented in Supplementary Table S3.
The units were standardized and are presented as Mg C ha−1 and Tg C for above and soil stocks and for the total C ecosystem stock. Data is shown as mean ±1 standard deviation (SD). When it was only possible to estimate soil or aboveground stocks for seagrass and saltmarshes of an ecoregion, we used mean values for the adjacent ecoregion to complete the estimate.
Accumulation rates
Data on soil accumulation rates from cores derived by means of 210Pb (last century) were compiled. Concentration profiles of 210Pb along the sediment cores were determined by alpha spectrometry through the measurement of 210Po after acid digestion of the samples, assuming radioactive equilibrium between the 210Pb and 210Po radionuclides. Gamma spectrometry measurements were also conducted in cores using germanium high purity detectors.
Studies of saltmarshes in the region lacked data for soil accumulation rates, and therefore, a conservative estimate of the global soil C accumulation rate was used. Ouyang and Lee75 reported a mean global rate of 2.4 Mg C ha−1 yr−1, with 5th and 95th percentiles of 0.41 and 7.36 Mg C ha−1 yr−1, based on studies that did not include any data on C burial rates from the Southwestern Atlantic coast. These estimates included several data outliers as well as being positively skewed towards low values. To derive a conservative estimate for the soil accumulation rate in the SWA, the geometric mean of the data, with outliers removed, was used (1.2 Mg C ha−1 yr−1).
Only 2 estimates of the C accumulation rate (0.11 and 0.34 Mg C ha−1 yr−1) in seagrass soils were available from the Southwestern Atlantic coast and both were derived from Ruppia maritima stands45. Considering the lack of data for seagrass meadows, the same approach taken for saltmarsh was employed using data from a review on the distribution of global C burial rates in seagrass soils76. An OC accumulation of 0.24 Mg C ha−1 yr−1 (95% C.I. 0.2–0.3 Mg C ha−1 yr−1) represented the geometric mean of seagrass sites where there was the measurable net accumulation rate was used to estimate the total accumulation of C in the SWA.
For mangroves, we were able to identify a relatively limited number of studies (n = 13) where the sedimentation rates had been estimated, including a total of 33 soil cores. Only a few of these studies reported CAR, including a total of 18 soil cores, ranging from 0.72 to 10.2 Mg C ha−1 yr−1, with an average of 2.6 Mg C ha−1 yr−1. CAR estimates for the other regions were calculated based on average sedimentation rates and C contents in soils from each ecoregion.
Statistical analysis
Homogeneity of variances was examined using Cochran’s test. For the variables that followed a normal distribution (Shapiro–Wilk test) a one-way ANOVA and Tukey test were used to test for significant differences in these variables between treatments. Nonparametric tests (Kruskal–Wallis and Dunn’s test) were used to assess the differences for variables that were not normally distributed. Significance was tested at the 0.05 level (i.e., confidence interval at 95%). All tests were performed using the XRealStats package of Excel.
Data availability
The data underlying the research presented in our paper is available in the Supplementary Information and have been deposited in the Mendeley Data (Hatje, Vanessa (2023), “Hatje et al_2023_COMMSENV”, Mendeley Data, V1, https://doi.org/10.17632/schrbgywj9.1).
References
Nellemann, C. et al. Blue Carbon: A Rapid Response Assessment. United Nations Environment Programme (GRID-Arendal, 2009).
Lovelock, C. E. & Duarte, C. M. Dimensions of blue carbon and emerging perspectives. Biol. Lett. 15, 20180781 (2019).
McLeod, E. et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, 552–560 (2011).
Duarte, C., Losada, I., Hendriks, I., Mazarrasa, I. & Marba, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 3, 961–968 (2013).
Pendleton, L. et al. Estimating global ‘Blue Carbon’ emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 7, e43542 (2012).
Howard, J. et al. Clarifying the role of coastal and marine systems in climate mitigation. Front. Ecol. Environ. 42–50 https://doi.org/10.1002/fee.1451 (2017).
Reynolds, L. K., Waycott, M., McGlathery, K. J. & Orth, R. J. Ecosystem services returned through seagrass restoration. Restor. Ecol. 24, 583–588 (2016).
Das, S. Ecological restoration and livelihood: contribution of planted mangroves as nursery and habitat for artisanal and commercial fishery. World Dev. 94, 492–502 (2017).
Wheaton, J. M., Gibbins, C., Wainwright, J., Larsen, L. & McElroy, B. Preface: multiscale feedbacks in ecogeomorphology. Geomorphology 126, 265–268 (2011).
Rovai, A. S. et al. Global controls on carbon storage in mangrove soils. Nat. Clim. Chang. 8, 534 (2018).
Rovai, A. S. et al. Scaling mangrove aboveground biomass from site-level to continental-scale. Glob. Ecol. Biogeogr. 25, 286–298 (2015).
Sexton, J. O. et al. Conservation policy and the measurement of forests. Nat. Clim. Chang. 6, 192–196 (2016).
IPCC. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands, Published: IPCC, Switzerland. (IPCC, 2014).
Silva Junior, C. H. L. et al. The Brazilian Amazon deforestation rate in 2020 is the greatest of the decade. Nat. Ecol. Evol. 5, 144–145 (2021).
Giri, C. et al. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 20, 154–159 (2011).
Martinetto, P., Montemayor, D. I., Alberti, J., Costa, C. S. B. & Iribarne, O. Crab bioturbation and herbivory may account for variability in carbon sequestration and stocks in south west atlantic salt marshes. Front. Mar. Sci. 3, 1–12 (2016).
Copertino, M. S. et al. Seagrass and Submerged Aquatic Vegetation (VAS) habitats off the coast of Brazil: state of knowledge, conservation and main threats. Brazilian J. Oceanogr. 64, 53–80 (2016).
Simard, M., Pinto, N., Fisher, J. B. & Baccini, A. Mapping forest canopy height globally with spaceborne lidar. J. Geophys. Res. Biogeosci. 116, 1–12 (2011).
Hutchison, J., Manica, A., Swetnam, R., Balmford, A. & Spalding, M. Predicting global patterns in mangrove forest biomass. Conserv. Lett. 7, 233–240 (2014).
Macreadie, P. I. et al. Blue carbon as a natural climate solution. Nat. Rev. Earth Environ. 2, 0123456789 (2021).
Adame, M. et al. Future carbon emissions from global mangrove forest loss. Glob. Chang. Biol. 27, 2856–2866 (2021).
Goldberg, L., Lagomasino, D., Thomas, N. & Fatoyinbo, T. Global declines in human-driven mangrove loss. Glob. Chang. Biol. 26, 5844–5855 (2020).
ICMBio. Atlas dos Manguezais do Brasil. (ICMBio, 2018).
Short, F., Carruthers, T., Dennison, W. & Waycott, M. Global seagrass distribution and diversity: a bioregional model. J. Exp. Mar. Bio. Ecol. 350, 3–20 (2007).
Mckenzie, L. J. et al. The global distribution of seagrass meadows information for data reuse ecosystems OPEN ACCESS. Environ. Res. Lett. 15, 074041 (2020).
Charpy-Roubaud, C. & Sournia, A. The comparative estimation of phytoplanktonic and microphytobenthic production in the oceans. Mar. Microb. Food Webs. 4, 31–57 (1990).
Jayathilake, D. R. M. & Costello, M. J. A modelled global distribution of the seagrass biome. Biol. Conserv. 226, 120–126 (2018).
Schaeffer-Novelli, Y. et al. Climate changes in mangrove forests and salt marshes. Brazilian J. Oceanogr. 64, 37–52 (2016).
Isacch, J. et al. Distribution of saltmarsh plant communities associated with environmental factors along a latitudinal gradient on the south-west Atlantic coast. J. Biogeogr. 33, 888–900 (2006).
Serrano, O. et al. Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nat. Commun. 1–10 https://doi.org/10.1038/s41467-019-12176-8 (2019).
Fourqurean, J. W. et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 1–5 https://doi.org/10.1038/NGEO1477 (2012).
Atwood, T. B. et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 7, 523–528 (2017).
Jardine, S. L. & Siikamäki, J. V. A global predictive model of carbon in mangrove soils. Environ. Res. Lett. 9, 1–9 (2014).
Payne, R. J. et al. Peatland initiation and carbon accumulation in the Falkland Islands. Quat. Sci. Rev. 212, 213–218 (2019).
Nóbrega, G. N. et al. Edaphic factors controlling summer (rainy season) greenhouse gas emissions (CO2 and CH4) from semiarid mangrove soils (NE-Brazil). Sci. Total Environ. 542, 685–693 (2016).
Kauffman, J. et al. Carbon stocks of mangroves and salt marshes of the Amazon region, Brazil. Biol. Lett. 14, 1–13 (2018).
Santos-Andrade, M., Hatje, V., Arias-Ortiz, A., Patire, V. F. & da Silva, L. A. Human disturbance drives loss of soil organic matter and changes its stability and sources in mangroves. Environ. Res. 202, 111663 (2021).
Rovai, A. S., Riul, P., Twilley, R. R. & Rica, C. Scaling mangrove aboveground biomass from site-level to continental-scale. Glob. Ecol. Biogeogr. 25, 286–298 (2016).
Howard, J. L., Creed, J. C., Aguiar, M. V. P. & Fouqurean, J. W. CO2 released by carbonate sediment production in some coastal areas may offset the benefits of seagrass “Blue Carbon” storage. Limnol. Oceanogr. https://doi.org/10.1002/lno.10621 (2017).
Mazarrasa, I. et al. Factors determining seagrass blue carbon across bioregions and geomorphologies. Global Biogeochem. Cycles 35, 1–17 (2021).
Mazarrasa, I. et al. Effect of environmental factors (wave exposure and depth) and anthropogenic pressure in the C sink capacity of Posidonia oceanica meadows. Limnol. Oceanogr. 62, 1436–1450 (2017).
Mazarrasa, I. et al. Habitat characteristics provide insights of carbon storage in seagrass meadows. Mar. Pollut. Bull. 134, 106–117 (2018).
Ford, H. et al. Large-scale predictions of salt-marsh carbon stock based on simple observations of plant community and soil type. Biogeosciences 16, 425–436 (2019).
Gorham, C., Lavery, P., Kelleway, J. J., Salinas, C. & Serrano, O. Soil carbon stocks vary across geomorphic settings in Australian temperate tidal marsh ecosystems. Ecosystems 24, 319–334 (2021).
Patterson, E. Stocks and Sources of Carbon Buried in the Salt Marshes and Seagrass Beds of Patos Lagoon, Southern Brazil (Bates College, 2016).
Breithaupt, J. L., Smoak, J. M., Iii, T. J. S., Sanders, C. J. & Hoare, A. Organic carbon burial rates in mangrove sediments: strengthening the global budget. Glob. Biogeochem. Cycle. 26, 1–11 (2012).
Arias-Ortiz, A. et al. Losses of soil organic carbon with deforestation in mangroves of Madagascar. Ecosystems 24, 1–19 (2020).
Rovai, A. S. et al. Macroecological patterns of forest structure and allometric scaling in mangrove forests. Glob. Ecol. Biogeogr. 00, 1–14 (2021).
Rovai, A. S., Twilley, R. R., Worthington, T. A., Riul, P. & Worthington, T. A. Brazilian mangroves: blue carbon hotspots of national and global relevance to natural climate solutions. Front. For. Glob. Chang. 4, 7875333 (2022).
Hatje, V., Masqué, P., Patire, V. F., Dórea, A. & Barros, F. Blue carbon stocks, accumulation rates, and associated spatial variability in Brazilian mangroves. Limnol. Oceanogr. 66, 321–334 (2021).
Young, M. A. et al. Optimal soil carbon sampling designs to achieve cost-effectiveness: a case study in blue carbon ecosystems. Biol. Lett. 14, 20180416 (2018).
Sanderman, J. et al. A global map of mangrove forest soil carbon at 30 m spatial resolution wetland greenhouse gas inventorying OPEN ACCESS A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett. 13, 055002 (2018).
Kauffman, J. et al. Global change biology Carbon stocks of mangroves and salt marshes of the Amazon region, Brazil. Biol. Lett. 14, 20180208 (2018).
Kauffman, J. B. et al. Total ecosystem carbon stocks of mangroves across broad global environmental and physical gradients. Ecol. Monogr. 90, e01405 (2020).
Bouza, P., Ríos, I., Rostagno, C. M. & Saín, C. in Late Cenozoic of Península Valdés 161–190 (Springer, 2017).
Howard, J. L., Creed, J. C., Aguiar, M. V. P. & Fourqurean, J. W. CO2 released by carbonate sediment production in some coastal areas may offset the benefits of seagrass “Blue Carbon” storage. Limnol. Oceanogr. 160–172 https://doi.org/10.1002/lno.10621 (2018).
Otero, X. L. et al. High fragility of the soil organic C pools in mangrove forests. Mar. Pollut. Bull. 119, 460–464 (2017).
MapBiomas. Projeto MapBiomas–Coleção 5 da Série Anual de Mapas de Cobertura e Uso de Solo do Brasil. Available at: https://mapbiomas.org. (2020).
Santos, D. et al. First assessment of carbon stock in the belowground biomass of Brazilian mangroves. An. Acad. Bras. Cienc. 89, 1579–1589 (2017).
Diniz, C. et al. Brazilian mangrove status: three decades of satellite data analysis. Remote Sens. 11, 1–19 (2019).
Godoy, M. D. P. & De Lacerda, L. D. Mangroves response to climate change: a review of recent findings on mangrove extension and distribution. An. Acad. Bras. Cienc. 87, 651–667 (2015).
Kauffman, J. et al. Shrimp ponds lead to massive loss of soil carbon and greenhouse gas emissions in northeastern Brazilian mangroves. Ecol. Evol. 8, 5530–5540 (2018).
Zeng, Y., Friess, D. A., Sarira, T. V., Siman, K. & Koh, L. P. Global potential and limits of mangrove blue carbon for climate change mitigation. Curr. Biol. 31, 1737–1743.e3 (2021).
Servino, R. N., Eduardo, L., Gomes, D. O. & Bernardino, A. F. Extreme weather impacts on tropical mangrove forests in the Eastern Brazil Marine Ecoregion. Sci. Total Environ. 628–629, 233–240 (2018).
Bacino, G. L., Dragani, W. C. & Codignotto, J. O. Changes in wave climate and its impact on the coastal erosion in Samborombón Bay, Río de la Plata estuary, Argentina. Estuar. Coast. Shelf Sci. 219, 71–80 (2019).
Short, F. et al. SeagrassNet monitoring across the Americas: case studies of seagrass decline. Mar. Ecol. 27, 277–289 (2006).
Gomes, L. E., de, O., Vescovi, L. C. & Bernardino, A. F. The collapse of mangrove litterfall production following a climate-related forest loss in Brazil. Mar. Pollut. Bull. 162, 111910 (2021).
Cohen, M. C. L. et al. Southward migration of the Austral limit of mangroves in South America. CATENA 195, 104775 (2020).
Spalding, M. D. et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Biosciences 57, 573–583 (2007).
Howard, J., Hoyt, S., Isensee, K., Pidgeon, E. & Telszewski, M. Coastal Blue Carbon: Methods for Assessing Carbon Stocks and Emissions Factors in Mangroves, Tidal Salt Marshes, and Seagrass Meadows (UNESCO 2014).
Negrin, V. L., de Villalobos, A. E., Trilla, G. G., Botté, S. E. & Marcovecchio, J. E. Above- and belowground biomass and nutrient pools of Spartina alterniflora (smooth cordgrass) in a South American salt marsh. Chem. Ecol. 28, 391–404 (2012).
Ruehlmann, J. & Körschens, M. Calculating the effect of soil organic matter concentration on soil bulk density. Soil Sci. Soc. Am. J. 73, 876–885 (2009).
UNEP-WCMC & Short, F. Global Distribution of Seagrasses (version 7.1). Seventh Update to the Data Layer Used in Green and Short (2003) (UN Environment Programme World Conservation Monitoring Centre, 2021).
Mcowen, C. J. et al. A global map of saltmarshes. Biodivers. Data J. 5, e11764 (2017).
Ouyang, X. & Lee, S. Y. Updated estimates of carbon accumulation rates in coastal marsh sediments. Biogeosciences 11, 5057–5071 (2014).
Arias-Ortiz, A. Carbon Sequestration Rates in Coastal Blue Carbon Ecosystems: A Perspective on Climate Change Mitigation (Universitat Autonoma de Barcelona, 2019).
Hamilton, S. E. & Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Global Ecol. Biogeogr. 25, 729–738 (2016).
Acknowledgements
This work was supported by CNPq (441264/2017-4, 441492/2016-9, 486676/2011-0) and CNPq/ FINEP (Rede CLIMA 01.13.0353-00). The authors were sponsored by CAPES (VFP, Finance Code 001) and CNPq (V.H., 304823/2018-0). We thank G. N. Nobrega for kindly providing seagrass DBD; to E. Paterson and P. Dostie for collection and analysis of soils. M.C., H.K., and B.J are grateful to The Blue Carbon Initiative. The IAEA is grateful for the support provided to its Environment Laboratories by the Government of the Principality of Monaco.
Author information
Authors and Affiliations
Contributions
V.H., J.C.C., M.S.C. conceived, designed the research, coordinated data acquisition, and drafted the original manuscript. V.H., J.C.C., M.C., V.F.P., P.M., H.K. analyzed the data, drafted, and edited the manuscript. V.H., M.C., V.F.P., X.O., J.O., B.J.J., H.K., P.M., and J.C.C. approved the final submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks the anonymous reviewers for their contribution to the peer review of this work. Primary handling editors: Annie Bourbonnais and Clare Davis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hatje, V., Copertino, M., Patire, V.F. et al. Vegetated coastal ecosystems in the Southwestern Atlantic Ocean are an unexploited opportunity for climate change mitigation. Commun Earth Environ 4, 160 (2023). https://doi.org/10.1038/s43247-023-00828-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-023-00828-z
- Springer Nature Limited
This article is cited by
-
Traversed dynamics of climate change and One Health
Environmental Sciences Europe (2024)
-
The inclusion of Amazon mangroves in Brazil’s REDD+ program
Nature Communications (2024)
-
The blue carbon of southern southwest Atlantic salt marshes and their biotic and abiotic drivers
Nature Communications (2023)
-
Global dataset of soil organic carbon in tidal marshes
Scientific Data (2023)