Abstract
Feeding behaviour is influenced by two primary factors: homoeostatic needs driven by hunger and hedonic desires for pleasure even in the absence of hunger. While efficient homoeostatic feeding is vital for survival, excessive hedonic feeding can lead to adverse consequences such as obesity and metabolic dysregulations. However, the neurobiological mechanisms that orchestrate homoeostatic versus hedonic food consumption remain largely unknown. Here we show that GABAergic proenkephalin (Penk) neurons in the diagonal band of Broca (DBB) of male mice respond to food presentation. We further demonstrate that a subset of DBBPenk neurons that project to the paraventricular nucleus of the hypothalamus are preferentially activated upon food presentation during fasting periods and transmit a positive valence to facilitate feeding. On the other hand, a separate subset of DBBPenk neurons that project to the lateral hypothalamus are preferentially activated when detecting a high-fat high-sugar (HFHS) diet and transmit a negative valence to inhibit food consumption. Notably, when given free choice of chow and HFHS diets, mice with the whole DBBPenk population ablated exhibit reduced consumption of chow but increased intake of the HFHS diet, resulting in accelerated development of obesity and metabolic disturbances. Together, we identify a molecularly defined neural population in male mice that is crucial for the maintenance of energy balance by facilitating homoeostatic feeding while suppressing hedonic overeating.





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article. No third-party materials were included in this paper. Source data are provided with this paper.
References
Finucane, M. M. et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377, 557–567 (2011).
Saper, C. B., Chou, T. C. & Elmquist, J. K. The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199–211 (2002).
Rossi, M. A. & Stuber, G. D. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab. 27, 42–56 (2018).
Berthoud, H. R., Munzberg, H. & Morrison, C. D. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 152, 1728–1738 (2017).
Kenny, P. J. Reward mechanisms in obesity: new insights and future directions. Neuron 69, 664–679 (2011).
Domenger, D. & Schwarting, R. K. The serial reaction time task in the rat: effects of D1 and D2 dopamine-receptor antagonists. Behav. Brain Res. 175, 212–222 (2006).
Nakamura, K., Nakamura, Y. & Kataoka, N. A hypothalamomedullary network for physiological responses to environmental stresses. Nat. Rev. Neurosci. 23, 35–52 (2022).
Cheng, W. et al. Hindbrain circuits in the control of eating behaviour and energy balance. Nat. Metab. 4, 826–835 (2022).
Waterson, M. J. & Horvath, T. L. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab. 22, 962–970 (2015).
Cassidy, R. M. et al. A lateral hypothalamus to basal forebrain neurocircuit promotes feeding by suppressing responses to anxiogenic environmental cues. Sci. Adv. 5, eaav1640 (2019).
Patel, J. M. et al. Sensory perception drives food avoidance through excitatory basal forebrain circuits. eLife https://doi.org/10.7554/eLife.44548 (2019).
Herman, A. M. et al. A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature 538, 253–256 (2016).
Swanson, J. L. et al. Activation of basal forebrain-to-lateral habenula circuitry drives reflexive aversion and suppresses feeding behavior. Sci. Rep. 12, 22044 (2022).
Roman-Ortiz, C., Guevara, J. A. & Clem, R. L. GABAergic basal forebrain projections to the periaqueductal gray promote food consumption, reward and predation. Sci. Rep. 11, 22638 (2021).
Cai, J. et al. An excitatory projection from the basal forebrain to the ventral tegmental area that underlies anorexia-like phenotypes. Neuron https://doi.org/10.1016/j.neuron.2023.11.001 (2023).
Sukhov, R. R., Walker, L. C., Rance, N. E., Price, D. L. & Young, W. S. 3rd Opioid precursor gene expression in the human hypothalamus. J. Comp. Neurol. 353, 604–622 (1995).
Anand, J. P. & Montgomery, D. Multifunctional opioid ligands. Handb. Exp. Pharmacol. 247, 21–51 (2018).
Bodnar, R. J. Endogenous opioid modulation of food intake and body weight: Implications for opioid influences upon motivation and addiction. Peptides 116, 42–62 (2019).
Janecka, A., Fichna, J. & Janecki, T. Opioid receptors and their ligands. Curr. Top. Med. Chem. 4, 1–17 (2004).
Ostlund, S. B., Kosheleff, A., Maidment, N. T. & Murphy, N. P. Decreased consumption of sweet fluids in mu opioid receptor knockout mice: a microstructural analysis of licking behavior. Psychopharmacology 229, 105–113 (2013).
Mendez, I. A., Ostlund, S. B., Maidment, N. T. & Murphy, N. P. Involvement of endogenous enkephalins and beta-endorphin in feeding and diet-induced obesity. Neuropsychopharmacology 40, 2103–2112 (2015).
Castro, D. C. et al. An endogenous opioid circuit determines state-dependent reward consumption. Nature 598, 646–651 (2021).
Sternson, S. M. & Eiselt, A. K. Three pillars for the neural control of appetite. Annu. Rev. Physiol. 79, 401–423 (2017).
Sweeney, P. & Yang, Y. Neural circuit mechanisms underlying emotional regulation of homeostatic feeding. Trends Endocrinol. Metab. 28, 437–448 (2017).
Wu, Z. et al. GABAergic projections from lateral hypothalamus to paraventricular hypothalamic nucleus promote feeding. J. Neurosci. 35, 3312–3318 (2015).
Evers, C., Marijn Stok, F. & de Ridder, D. T. Feeding your feelings: emotion regulation strategies and emotional eating. Personal. Soc. Psychol. Bull. 36, 792–804 (2010).
Qin, C., Li, J. & Tang, K. The paraventricular nucleus of the hypothalamus: development, function, and human diseases. Endocrinology 159, 3458–3472 (2018).
Zingg, B. et al. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47 (2017).
Jennings, J. H., Rizzi, G., Stamatakis, A. M., Ung, R. L. & Stuber, G. D. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521 (2013).
Petrovich, G. D. Lateral hypothalamus as a motivation-cognition interface in the control of feeding behavior. Front. Syst. Neurosci. 12, 14 (2018).
Ferrario, C. R. et al. Homeostasis meets motivation in the battle to control food intake. J. Neurosci. 36, 11469–11481 (2016).
Sutton, A. K. & Krashes, M. J. Integrating hunger with rival motivations. Trends Endocrinol. Metab. 31, 495–507 (2020).
Ahn, B. H., Kim, M. & Kim, S. Y. Brain circuits for promoting homeostatic and non-homeostatic appetites. Exp. Mol. Med. 54, 349–357 (2022).
Jais, A. et al. PNOC(ARC) neurons promote hyperphagia and obesity upon high-fat-diet feeding. Neuron 106, 1009–1025 e1010 (2020).
Shin, S. et al. Early adversity promotes binge-like eating habits by remodeling a leptin-responsive lateral hypothalamus-brainstem pathway. Nat. Neurosci. 26, 79–91 (2023).
Xu, Y., Elmquist, J. K. & Fukuda, M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann. NY Acad. Sci. 1243, 1–14 (2011).
Liu, H. et al. Hypothalamic Grb10 enhances leptin signalling and promotes weight loss. Nat. Metab. 5, 147–164 (2023).
Liu, T., Xu, Y., Yi, C. X., Tong, Q. & Cai, D. The hypothalamus for whole-body physiology: from metabolism to aging. Protein Cell 13, 394–421 (2022).
Kim, E. R. et al. Paraventricular hypothalamus mediates diurnal rhythm of metabolism. Nat. Commun. 11, 3794 (2020).
An, J. J., Liao, G. Y., Kinney, C. E., Sahibzada, N. & Xu, B. Discrete BDNF neurons in the paraventricular hypothalamus control feeding and energy expenditure. Cell Metab. 22, 175–188 (2015).
Sutton, A. K., Myers, M. G. Jr. & Olson, D. P. The role of PVH circuits in leptin action and energy balance. Annu. Rev. Physiol. 78, 207–221 (2016).
Grzelka, K. et al. A synaptic amplifier of hunger for regaining body weight in the hypothalamus. Cell Metab. https://doi.org/10.1016/j.cmet.2023.03.002 (2023).
Krashes, M. J. et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242 (2014).
Garfield, A. S. et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 18, 863–871 (2015).
Li, C. et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 29, 681–694 e685 (2019).
Atasoy, D., Betley, J. N., Su, H. H. & Sternson, S. M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Vardy, E. et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86, 936–946 (2015).
Li, M. M. et al. The paraventricular hypothalamus regulates satiety and prevents obesity via two genetically distinct circuits. Neuron 102, 653–667 e656 (2019).
Qian, S. et al. A temperature-regulated circuit for feeding behavior. Nat. Commun. 13, 4229 (2022).
Xu, Y. et al. Identification of a neurocircuit underlying regulation of feeding by stress-related emotional responses. Nat. Commun. 10, 3446 (2019).
Mangieri, L. R. et al. A neural basis for antagonistic control of feeding and compulsive behaviors. Nat. Commun. 9, 52 (2018).
Delgado, J. M. & Anand, B. K. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am. J. Physiol. 172, 162–168 (1953).
Knight, Z. A. et al. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell 151, 1126–1137 (2012).
Jennings, J. H. et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527 (2015).
Fu, O. et al. Hypothalamic neuronal circuits regulating hunger-induced taste modification. Nat. Commun. 10, 4560 (2019).
Lee, J., Raycraft, L. & Johnson, A. W. The dynamic regulation of appetitive behavior through lateral hypothalamic orexin and melanin concentrating hormone expressing cells. Physiol. Behav. 229, 113234 (2021).
Burdakov, D. & Karnani, M. M. Ultra-sparse connectivity within the lateral hypothalamus. Curr. Biol. 30, 4063–4070 e4062 (2020).
Phua, S. C. et al. A distinct parabrachial-to-lateral hypothalamus circuit for motivational suppression of feeding by nociception. Sci. Adv. https://doi.org/10.1126/sciadv.abe4323 (2021).
Schneeberger, M. et al. Pharmacological targeting of glutamatergic neurons within the brainstem for weight reduction. Nat. Metab. 4, 1495–1513 (2022).
Denis, R. G. P. et al. Palatability can drive feeding independent of AgRP neurons. Cell Metab. 25, 975 (2017).
Meye, F. J. & Adan, R. A. Feelings about food: the ventral tegmental area in food reward and emotional eating. Trends Pharmacol. Sci. 35, 31–40 (2014).
Christoffel, D. J. et al. Input-specific modulation of murine nucleus accumbens differentially regulates hedonic feeding. Nat. Commun. 12, 2135 (2021).
Stuber, G. D. & Wise, R. A. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci. 19, 198–205 (2016).
Nieh, E. H. et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90, 1286–1298 (2016).
Patterson, C. M. et al. Ventral tegmental area neurotensin signaling links the lateral hypothalamus to locomotor activity and striatal dopamine efflux in male mice. Endocrinology 156, 1692–1700 (2015).
Linders, L. E. et al. Stress-driven potentiation of lateral hypothalamic synapses onto ventral tegmental area dopamine neurons causes increased consumption of palatable food. Nat. Commun. 13, 6898 (2022).
Fadel, J. & Burk, J. A. Orexin/hypocretin modulation of the basal forebrain cholinergic system: role in attention. Brain Res. 1314, 112–123 (2010).
Henny, P. & Jones, B. E. Vesicular glutamate (VGlut), GABA (VGAT), and acetylcholine (VACht) transporters in basal forebrain axon terminals innervating the lateral hypothalamus. J. Comp. Neurol. 496, 453–467 (2006).
Fuente Gonzalez, C. E. et al. Relationship between emotional eating, consumption of hyperpalatable energy-dense foods, and indicators of nutritional status: a systematic review. J. Obes. 2022, 4243868 (2022).
Betley, J. N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015).
Douglass, A. M. et al. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci. 20, 1384–1394 (2017).
Cai, X. et al. A D2 to D1 shift in dopaminergic inputs to midbrain 5-HT neurons causes anorexia in mice. Nat. Neurosci. 25, 646–658 (2022).
He, Y. et al. 5-HT recruits distinct neurocircuits to inhibit hunger-driven and non-hunger-driven feeding. Mol. Psychiatry 26, 7211–7224 (2021).
Liu, H. et al. TPH2 in the dorsal Raphe nuclei regulates energy balance in a sex-dependent manner. Endocrinology https://doi.org/10.1210/endocr/bqaa183 (2021).
Nogi, Y. et al. Expression of feeding-related neuromodulatory signalling molecules in the mouse central olfactory system. Sci. Rep. 10, 890 (2020).
Murata, K. Hypothetical roles of the olfactory tubercle in odor-guided eating behavior. Front. Neural Circuits 14, 577880 (2020).
Acknowledgements
The investigators were supported by grants from the USDA/CRIS (51000-064-01S to Y.X. and 3092-51000-062-04(B)S to C.W.), Texas Children’s Research Scholar funds (ACCT 3410 to Y.H.), American Heart Association (23POST1030352 to Hailan Liu) and National Institutes of Health NIDDK (1R01DK138123 and 1R01DK138518 to Y.X. and 1F32DK134121-01A1 to K.M.C.).
Author information
Authors and Affiliations
Contributions
Hailan Liu was involved in the experimental design and most of procedures, data acquisition and analyses, and wrote the manuscript. Y.H. conducted electrophysiological experiments and data analysis. J.C.B. performed PCA analysis on the GRIN lens data. Y.L., M.Y., O.Z.G., K.M.C., M.W., X.F., Hesong Liu, L.T., N.Y. and J.H. contributed to the generation of study mice and data discussion. Y.Y., Q.T., B.R.A. and C.W. were involved in study design and data discussion. Y.X. conceptualized and designed this study. Y.X. and Y.H. supervised this work, and as such, had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Yu Fu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
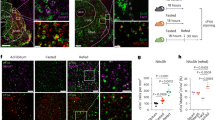
Extended Data Fig. 1 DBBPenk neurons are activated by inaccessible food.
a-c. GCaMP6m signals in DBBPenk neurons of male mice in response to inaccessible chow (a) or HFHS (b) diet or non-food object (c) after an overnight fast (n = 6). Time 0 marks the moment when mice detect food or object. d. GCaMP6m signals in DBBPenk neurons of overnight fasted male mice when they are biting chow food. Time 0 marks the moment when mice start to bite food. e. A representative trace showing the response of DBBPenk neurons when mice are approaching or biting chow diet. f. GCaMP6m signals in DBBPenk neurons of sated male mice when they are biting HFHS food. Time 0 marks the moment when mice start to bite food. g. A representative trace showing the response of DBBPenk neurons when mice are approaching or biting HFHS diet. Data are expressed as mean ± SEM.
Extended Data Fig. 2 PCA classification of DBBPenk neurons.
The principal-component analysis classifies the responses of DBBPenk neurons into distinct reaction patterns.
Extended Data Fig. 3 DBBPenk neurons are activated by inaccessible food and tail suspension.
a. Traces showing the mean alterations in GCaMP6m fluorescence across 5 different types (left panel) and changes in calcium fluorescence of 65 neurons (right panel) from 6 food-deprived male mice in the presence of inaccessible chow diets. b. Bar graph showing the area under the curve for a. c. Traces showing the mean alterations in GCaMP6m fluorescence across 5 different types (left panel) and changes in calcium fluorescence of 65 neurons (right panel) from 6 food-deprived male mice in the presence of inaccessible HFHS diets. d. Bar graph showing the area under the curve for c. e. Traces showing the mean alterations in GCaMP6m fluorescence across 5 different types (left panel) and changes in calcium fluorescence of 65 neurons (right panel) from 6 food-deprived male mice in the presence of non-food object. f. Bar graph showing the area under the curve for e. g. Traces showing the mean alterations in GCaMP6m fluorescence across 5 different types (left panel) and changes in calcium fluorescence of 65 neurons (right panel) from 6 satiated male mice in the presence of inaccessible HFHS diet. h. Bar graph showing the area under the curve for g. i. Traces showing the mean alterations in GCaMP6m fluorescence across 5 different types (left panel) and changes in calcium fluorescence of 65 neurons (right paenl) from 6 satiated male mice in response to a 5 s tail suspension. Time 0 marks the start of food approaching for a, c, e, g or tail suspension for i. j. Bar graph showing the area under the curve for i. n = 20 for type 1, 13 for type 2, 16 for type 3, 5 for type 4, 11 for type 5 for b, d, f, h, j. The identical cell possesses the same ID to facilitate the comparison. Data are expressed as mean ± SEM or individual data points. One-way ANOVA with Sidak’s post hoc analysis (b, d, f, h, j).
Extended Data Fig. 4 DBBPenk neurons are exclusively GABAergic.
a. Representative immunofluorescence images showing the expression of tdTomato and ChAT in the DBB of 3 Penk-ires2-Cre/Rosa26-LSL-tdTomato mice. b. Representative RNAscope images showing Penk, Vgat and Vglut2 mRNA in the DBB of Penk-ires2-Cre/Rosa26-LSL-tdTomato mice. c. The number of Penk+, Vgat+, Vglut2+ cells in the DBB (n = 3). d. The percentage of Penk-expressing GABA neurons in the DBB. Data are expressed as mean ± SEM or individual data points.
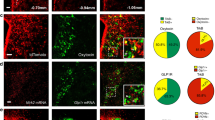
Extended Data Fig. 5 Projections from DBBPenk neurons.
a. Scheme for Cre-dependent ChR2 injection into the DBB of Penk-ires2-Cre mice. b. Representative images showing ChR2 expression in the DBB and EYFP-labelled fibres in the PVH, LH, OB, SuM, hippocampus and DRN of 3 male Penk-ires2-Cre mice.
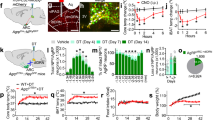
Extended Data Fig. 6 PVH-projecting DBBPenk neurons are activated by homoeostatic needs and stress.
a. The number of DBBPenk neurons that project to the PVH. b. mCherry and GFP-labelled cell bodies in the DBB. c. Quantification of mCherry+ and GFP+ cells in the DBB. d. Representative image showing GCaMP6m expression in PVH-projecting DBBPenk neurons for 3 mice. e-f. GCaMP6m signals in PVH-projecting DBBPenk neurons in overnight fasted male mice in response to inaccessible chow (e) or HFHS (f) diets (n = 6). g. GCaMP6m signals in PVH-projecting DBBPenk neurons in satiated male mice in response to inaccessible HFHS diet (n = 6). h. GCaMP6m signals in PVH-projecting DBBPenk neurons in overnight fasted male mice in response to non-food object (n = 6). i-j. GCaMP6m signals in PVH-projecting DBBPenk neurons in satiated male mice in response to a 5 s tail suspension (i) and TMT exposure (j, n = 5). Time 0 marks the start of item detection (e-h) or treatment (i-j). k-l. GCaMP6m signals in PVH-projecting DBBPenk neurons in overnight fasted male mice that were pretreated with HFD for 4 weeks in response to chow (k) or high-fat (l) diets (n = 5). Time 0 marks the moment when mice detect food. m. GCaMP6m signals in PVH-projecting DBBPenk neurons in satiated male mice treated with HFD for 4 weeks in response to a 5 s tail suspension (n = 5). Time 0 marks the start of tail suspension. n-o. Total travel distance (n) and number of entries into the light-paired chamber (o) in male control and ChR2 expressing mice at various conditions (n = 7 per group). p. Representative RNAscope images showing Penk mRNA in the DBB of wild-type and Penk-ires2-Cre mice that had received retrograde Cre-dependent Flp into the PVH and Flp-dependent DTA injection into the DBB. q. Quantification of Penk+ neurons in the DBB of wild-type (n = 3) and Penk-ires2-Cre (n = 3) mice. Data are expressed as mean ± SEM or individual data points. Two-tailed unpaired Student’s t test (q) or one-way ANOVA with Sidak’s post hoc analysis (n-o).
Extended Data Fig. 7 LH-projecting DBBPenk neurons are activated by hedonic demands and stress.
a. The number of DBBPenk neurons that project to the LH. b. Representative image showing GCaMP6m expression in LH-projecting DBBPenk neurons. c-d. GCaMP6m signals in LH-projecting DBBPenk neurons in overnight fasted male mice in response to inaccessible chow (c) or HFHS (d) diets (n = 6). e. GCaMP6m signals in LH-projecting DBBPenk neurons in satiated male mice in response to inaccessible HFHS diet (n = 6). f. GCaMP6m signals in LH-projecting DBBPenk neurons in overnight fasted male mice in response to non-food object (n = 6). g-h. GCaMP6m signals in LH-projecting DBBPenk neurons in satiated male mice in response to high-fat diet containing either 30% (g) or 60% (h) fat content (n = 5). i. Area under curve for g and h. j-k. GCaMP6m signals in LH-projecting DBBPenk neurons in satiated male mice in response to a 5 s tail suspension (j) and TMT exposure (k, n = 5). Time 0 marks the start of item detection (e-h) or treatment (j-k). l-m. GCaMP6m signals in LH-projecting DBBPenk neurons in satiated (l) or fasted (m) male mice that were pretreated with HFD for 4 weeks in response to HFD (n = 5). Time 0 marks the moment when mice detect food. n. GCaMP6m signals in LH-projecting DBBPenk neurons in satiated male mice treated with HFD for 4 weeks in response to a 5 s tail suspension (n = 5). Time 0 marks the start of tail suspension. o-p. Total travel distance (o) and number of entries into the light-paired chamber (p) in male control and ChR2 expressing mice at various conditions (n = 7 per group). q. Representative RNAscope images showing Penk mRNA in the DBB of wild-type and Penk-ires2-Cre mice that had received retrograde Cre-dependent Flp into the LH and Flp-dependent DTA injection into the DBB. r. Quantification of Penk+ neurons in the DBB of wild-type (n = 3) and Penk-ires2-Cre (n = 3) mice. Data are expressed as mean ± SEM or individual data points. Two-tailed unpaired Student’s t test (i, r) or one-way ANOVA with Sidak’s post hoc analysis (o-p).
Extended Data Fig. 8 DBBPenk neurons are required to maintain energy homoeostasis.
a. Scheme for Cre-dependent DTR delivery into the DBB of Penk-ires2-Cre mice. b. Representative RNAscope images showing Penk mRNA in the DBB of wild-type and Penk-ires2-Cre mice who had both received Cre-dependent AAV encoding DTR injection into the DBB followed by DT treatment. c. Quantification of Penk+ neurons in the DBB of wild-type (n = 5) and Penk-ires2-Cre (n = 6) mice receiving AAV-DIO-DTR injection into the DBB. d-e. Cumulative chow intake (d) and body weight change (e) after DT injection in male control (n = 8) and DTR expressed (n = 7) mice. f. Total travel distance, centre entries, and centre duration in the open field box test in male control (n = 8) and DTR expressed (n = 7) mice. g. Total travel distance and open arm entries in the elevated plus maze test in male control (n = 8) and DTR expressed (n = 7) mice. h. Open arm duration in the elevated plus maze test in male control (n = 8) and DTR expressed (n = 7) mice. i-l. Chow (i), HFHS (j) and calorie intake (k) and body weight change (l) in male control (n = 8) and DTR expressed (n = 7) mice after DT injection, mice were provided with free access to chow and HFHS diet. m. Blood glucose level in control (n = 8) and DTR expressing (n = 7) mice 3 weeks after DT injection. n. Glucose tolerance test in male control (n = 8) and DTR expressing (n = 7) mice 4 weeks after DT injection. o. Area under curve for n. p. Insulin sensitivity test in male control (n = 8) and DTR expressed (n = 7) mice 5 weeks after DT injection. q. Area under curve for p. Data are expressed as mean ± SEM or individual data points. Two-tailed unpaired Student’s t test (c, f-h, m, o, q) or two-way ANOVA with Sidak’s post hoc analysis (d-e, i-l, n, p). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Bean, J.C., Li, Y. et al. Distinct basal forebrain-originated neural circuits promote homoeostatic feeding and suppress hedonic feeding in male mice. Nat Metab (2024). https://doi.org/10.1038/s42255-024-01099-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42255-024-01099-4
- Springer Nature Limited
This article is cited by
-
Neurons in the diagonal band of Broca moderate food intake
Nature Reviews Endocrinology (2024)