Abstract
Hair cortisol concentrations (HCC) are measured to assess long-term HPA-axis activity and may represent a valuable non-invasive tool to evaluate chronic stress in cats. This study investigated combing as a novel, low-stress method for HCC assessment, as well as possible associations between HCC and cat characteristics in 167 owned cats. Hair was sampled at veterinary clinics through clipping and/or combing the cat, or at home by the owner combing the cat. A questionnaire was sent to cat owners, including inquiries about the cat’s sex, health status, and exposure to stress. HCC was quantified using a commercial cortisol assay kit. Despite variations within and between sampling methods, Spearman’s correlation and Bland–Altman plots revealed a moderate correlation between clipped and combed samples (rs = 0.61, LOA -5.51 ± 22.54). In multiple linear regression, variations in HCC were observed based on sex, health status and cat group size. No associations were found between HCC and stress as assessed by owners. Despite study limitations and remaining uncertainty regarding factors influencing HCC, combing presents a convenient approach for evaluating long-term HPA-axis activity in clinical settings. The association between health and HCC suggests alterations in cortisol levels that are related to disease processes and stress-inducing events associated with the disease.
Similar content being viewed by others
Introduction
Chronic stress is a critical concern for animal welfare1,2,3, drawing increased attention in association with different physiological and behavioral alterations in cats4,5,6,7,8,9,10. Despite its significance to both cat and owner, signs of stress are frequently overlooked or underestimated by owners11. Stress is best estimated by combining behavioral and physiological data12,13, and as the hypothalamic–pituitary–adrenal (HPA) axis is central in stressor responses, measurement of HPA-axis activity is a common approach.
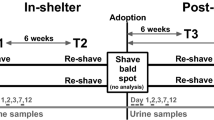
Over the past two decades, the use of hair cortisol concentration (HCC) to evaluate long-term HPA-axis activity has gained prominence14,15,16,17,18. HCC, reflecting cortisol incorporation into actively growing hair, is regarded as a reliable marker for long-term cortisol secretion in various species19. The benefits of using HCC include an easy and non-invasive sampling, convenient storage of hair20, and an excellent stability of cortisol in hair over time in investigated species18,20,21,22. Striving for a low-stress and convenient sampling method is beneficial for both cats and their owners. Traditionally, hair for analysis of cortisol concentration is sampled through shaving, often using the shave-reshave method, where hair is shaved at the beginning of the time frame of interest, and then shaved again after a period of regrowth23. Shed hair has been collected from sleeping nests24 and from fencing25.
However, our understanding of HCC has limitations, and recognizing them is essential, particularly when intending to evaluate animal welfare and stress. The mechanisms of cortisol incorporation into the hair are not fully understood23, and both hair and species-specific factors have to be considered when evaluating HCC18,22,23,24,26,27,28,29,30,31,32. In domestic cats this includes the varying distribution of primary and secondary hairs and seasonal hair follicular activity33,34. Higher HCC have been associated with litterbox issues35,36, aggressive behavior36,37, and neutering status in cats37, and both physiological and behavioral alterations have been suggested to affect cortisol concentrations in reproductively active females37,38. Lower HCC was measured in cats with well-kept hair coats compared to cats with poor hair coat conditions 35. An unkempt hair coat is reported in association with stress39 and with chronic diseases like hyperthyroidism and diabetes mellitus in cats40,41,42. However, when considering other factors, owner-reported chronic diseases in cats did not reveal significant associations with cortisol concentrations in either hair or nails35. Higher HCC were measured in shelter cats with dermatophyte infections43, but the only other factors available for data analysis were age and sex. This study aims to investigate a new low-stress hair sampling method in cats, building on previously used hair clipping techniques35,36,37,43,44, by combing the thoracic and abdominal regions. The objective was to develop a method that minimizes the need for sampling equipment, simplifies recruitment by lowering barriers for cat owners, and ensures low-stress handling of the cats. Another aim was to evaluate HCC in relation to owner-reported cat characteristics, health status, stressors, and signs of stress. We hypothesize that HCC from different sampling locations, sampled using different methods, will be positively correlated, and that HCC will be influenced by chronic disease and stress.
Materials and methods
Ethical approval
According to Swedish (SJVFS 2019:9, case no. L 150) and EU (EU Directive 2010/63) legislation, hair sampling by combing or clipping from privately owned cats does not require ethical permission. As required by L150, the owners received both written and oral information about the study and signed informed consent prior to inclusion. They were informed that they could withdraw their consent at any time. The experimental protocol was approved by The Board for Animals in Research and Teaching at The Swedish University of Agricultural Sciences. Data were handled in accordance with the General Data Protection Regulation. Hair samples were the only samples collected from the cats in this study, and all sampling took place in conjunction with veterinary appointments or by the owners at home. This study is reported in accordance with the ARRIVE guidelines45.
Study sample and questionnaire
Cats were recruited, and hair was sampled during veterinary visits to four participating veterinary clinics. Cats were also recruited through personal contacts, and in these cases, hair was sampled by the owners at home. Recruitment was limited to cats aged ≥ 6 months living in Sweden, whose owners consented to their participation. Participation was entirely voluntary, and no remuneration was provided. Information about the cats was obtained from a questionnaire. The exclusion criteria encompassed an incomplete questionnaire, contradictory information on health status of the cat, and the presence of acute injury, acute disease, or treatment with glucocorticoids 2–16 weeks before hair sampling.
Questions for the questionnaire were formulated taking into account previous studies and knowledge on stress in cats and the role of the owner in assessment3,7,11,46,47,48. The questions were assessed for comprehensibility and accurate interpretation by four cat owners, who were not part of the study. Based on the feedback from this pilot testing, the questionnaire was revised before digital distribution to participating cat owners via an online provider (Netigate), by e-mail, or, in case of missing e-mail, by mail.
Most questions were related to the time frame 2–16 weeks before hair sampling, roughly representative of HPA activity and cortisol incorporation in sampled hair strands. In addition, some questions concerned basic information about sex (male or female), the cat’s age at hair sampling, hair color and pattern. Further, two inquiries were about the owners’ perception about the cat’s quality of life and welfare. All owners had to answer the question “Is your cat healthy?” with answer options “Yes, my cat is healthy”, “Yes, my cat is healthy but has suffered from acute disease or injury”, “No, my cat has a chronic disease” and “No, my cat has a chronic disease and has suffered from acute disease or injury”. This formed the basis for grouping the cats according to their owner-reported health status. To assess the presence of stress, information was gathered on owner-reported exposure to potential stressors (e.g., new animal in household, house renovation, or cat relocation) and owner-observed signs of stress (e.g., house soiling, increased conflicts with other animals, changes in appetite or in activity levels). For cats with chronic disease, information on the presence of clinical signs related to the disease was gathered as a measure of disease impact on affected cats. For a summary of the questions and answer options, refer to Table 1. The answers from twenty-five questions were analyzed in this study.
Hair sampling
All hair sampling occurred between June 2020 and November 2022. Hair was sampled on one occasion from every cat. At least one hair sample was obtained from each cat: by clipping the dorsal area of the front leg or the caudoventral area of the abdomen, or by combing the dorsal back and lateral sides of the cat. Hair sampling was conducted either at veterinary visits (both clipping and combing) or by owners at home (combing only). For owners visiting veterinary clinics, paired samples were taken from more than one of the three different locations and methods. Clipping areas were selected for ease of retrieval, representing common venipuncture sites (dorsal area of the front leg) or areas prepared for abdominal ultrasound or surgery (the abdomen). This also ensured that no cat had to be clipped when not medically indicated, creating an opportunistic sampling process.
Detailed written instructions were provided to both veterinary staff and owners before sampling. Front leg and abdominal samples were obtained using well-cleaned mechanical clippers designed for small animals. The hair was cut at the level of the skin surface. For the front leg, a square of hair approximately 1.5 × 1.5 cm was cut from the dorsal area of the radius. Abdominal samples involved clipping hair from the caudoventral abdominal area in the anterior direction towards the costal arch, covering an area of approximately 5 × 10 cm, depending on the cat’s size. Instructions for combing included using a fine-toothed, cleaned comb on the dorsum and lateral area of the cat’s thorax and abdomen, and combing until an amount of hair equivalent to approximately 1 cm in diameter, when compressed, was achieved.
The hair samples were assigned a code for blinding, were placed in aluminum foil, and were stored at room temperature in a dark, dry place for a maximum time of 7 months before cortisol quantification.
Cortisol extraction
All laboratory work was performed by the research team, at the laboratory at the department of Clinical Sciences, Swedish University of Agricultural Sciences, Uppsala, Sweden. Based on previously measured hair growth rates in domestic cats34,49, the sampled hair was cut to a length of 40 mm, estimated to roughly represent the hair growth during 16 weeks. Extraction of cortisol from hair was performed and partly modified after Meyer et al.15, as follows: roughly 50 mg of hair was clipped into smaller parts with scissors and washed twice in 1.5 ml of isopropanol using a vortex mixer. When dried, excess hair was removed for a final hair weight of 50 mg. For samples containing hair < 50 mg, the lower weight was noted. In preparation for grinding the hair, three steel balls (Ø3.2 mm) were added to each sample and samples were deposited in liquid nitrogen for 4 min, increasing hair brittleness. Hair grinding was performed using a BeadBeater (5000 RPM, 6 × 30 s with a 30 s pause between sets). Freezing and grinding were repeated once. For cortisol extraction, 1.2 ml of methanol was added to the ground samples in a 22 h incubation phase on a laboratory rocker. After centrifugation, 0.6 ml of supernatant were extracted from each sample and then dried and reconstituted in a phosphate buffer (0.01 M PBS, pH 7.4). Reconstituted samples were stored frozen (-80°C) until cortisol quantification.
Assay performance and determination of cortisol concentration
For quantification of cortisol in hair, a commercial competitive high sensitivity ELISA kit (Salimetrics® Salivary Cortisol Enzyme Immunoassay Kit, LCC 2021) developed for quantitative measurement of human salivary cortisol and previously validated for analysis of cortisol in cat hair was used35. The minimum concentration of cortisol that could be distinguished from 0 was 0.007 µg/dL, and for samples with cortisol concentrations greater than 3.0 µg/dL, dilution was recommended. Results were converted from µg/dL to pg/mg hair15.
Hair from 13 cats and three 96-well plates were used to evaluate assay performance. Mean within-assay variation (CV) was determined on ten samples with low (2–3.6 pg/mg, three samples), medium (5.4–6.5 pg/mg, three samples) and high (8.7–85 pg/mg, four samples) cortisol concentrations, run in duplicates on one plate. Mean between-assay variation (CV) was determined by using three samples run in quadruplicates on three plates. For linearity and recovery upon dilution, one feline sample with high HCC was serially diluted with the assay diluent provided by the manufacturer 1:1, 1:1.33, 1:2, 1:4, 1:8, 1:16, and 1:32, and analyzed in triplicates on one plate. Recovery ([observed concentration/expected concentration]*100) was calculated. For recovery upon addition, three feline samples were spiked 1:1 in three different combinations (low and high sample, low and medium sample, low sample and high calibrator, as provided by the manufacturer) and analyzed in triplicates on one plate. To determine cortisol concentration, non-spiked samples from the three cats were also analyzed. Expected concentration ([concentration of non-spiked sample + concentration of the added sample or calibrator]*0.5) was calculated.
To evaluate stability of cortisol in cat hair over time, hair from two cats were prepared and the cortisol concentration was analyzed after 1, 8, and 24 months of storage in aluminum foil. For stability of extracted cortisol under deep frozen conditions, frozen reconstituted samples from the same two cats were re-run 8 and 24 months after initial cortisol analysis.
All individual samples were run in duplicates on a total of ten 96-well plates. In case of high CV (≥ 10%) between duplicates that could not be explained by low concentrations, samples were re-run. Samples with the lowest CV proceeded to data analysis. Samples exceeding the highest assay limit (3 µg/dL = 240 pg/mg) were set to 240 pg/mg.
Data analysis
In the case of missing or conflicting answers for any of the variables of interest, the respondent was contacted, and the answers were then adjusted, or the cat excluded accordingly. Cat age at the time of hair sampling was set to January 1st of each year. Cat sex was defined as spayed/neutered female or male, intact female or male, and females that, regardless of neutering status, had experienced pregnancy or estrus 2–16 weeks preceding hair sampling. For cats that were sampled in connection with neutering or spaying procedures, without experience of pregnancy or estrus, sex was set as intact. For owner-assessed stress, cats were assigned three different stress groups; no stress (no experience of potential stressors and no display of signs of stress), experience of potential stressors (but not displaying signs of stress), and displaying signs of stress. Cats that had experienced pregnancy or estrus were included in the group of cats displaying signs of stress, regardless of the owners’ assessment of stress signs. Cats in an owner-reported state of diabetic remission were included in the healthy group. For a summary of the questions and grouping of answers, see Table 1.
Data analysis was performed using R 50 and Minitab 51. Data was checked for normality using frequency distribution (histogram) and the Shapiro–Wilk normality test. Means and standard deviations were used when normality could be assumed, and median and inter-quartile ranges (IQR) were utilized when this was not the case. For evaluation of the relationship and differences of HCC between the three different hair sampling locations and methods, Spearman´s rank correlation and Bland–Altman plots were used, with 95% confidence intervals. Bland–Altman plots were interpreted graphically and by calculating the mean difference between paired values (bias). In cases where markedly differing datapoints, introducing a larger bias to the plot, were identified, they were removed prior to plotting. Limits of agreement (LOA, bias ± 1.96 SD) were constructed based on the standard variation (SD) of the differences between paired measurements. Multiple linear regression was used for evaluation of associations between HCC and owner reported cat characteristics, stress or health status. Two regression models were built; one with HCC quantified from clipped front leg samples as dependent variable, and one with HCC derived from combed samples as dependent variable. To improve model fit, the outcome variable HCC was log transformed for both regression models. Univariable linear regression was used for selection of variables, including variables with p < 0.2 in further model building. Based on previous studies35,43, the variables health status, presence of other animal species in the household, occurrence of potential stressors and signs of stress were included in building of the multivariable regression model, regardless of univariable regression outcome. The final regression model was decided with a backwards elimination process, where a lowered Akaike information criterion (AIC) was combined with exclusion of variables with p ≥ 0.05. Confidence intervals were set to 95% with a significance level of 5%. Quantile–quantile plots (QQ plots) and normality tests were used to assess regression residuals for normality. Biologically plausible interactions were included, and possible confounders were controlled in each regression model.
Results
Cats and general characteristics
In total 200 cats were recruited. Of these, 33 were excluded due to regular treatment with glucocorticoids, incomplete or obviously misinterpreted questionnaire answers, acute injury or disease, or contradictory information on health status. Complete questionnaires and hair from 167 cats remained for analysis (Fig. 1). One-hundred and three cats were reported as healthy, and 64 cats as chronically ill.
In healthy cats (n = 103), median age at sampling was 3 years (IQR 2–8). The majority of cats were spayed/neutered (63%), 15.5% were intact females, 5% were intact males, and 16.5% were females that, irrespective of neutering status at the time of sampling, had experienced pregnancy or a period of estrus.
In cats with chronic disease (n = 64), median age at hair sampling was 14 years (IQR 11–16). All cats were spayed or neutered, and no cat had experienced pregnancy or estrus. Type of chronic illness included one or more of the following conditions; diabetes mellitus (n = 28), hyperthyroidism (n = 19), chronic kidney disease (n = 14), hypertension (n = 5), arthritis (n = 3), chronic pancreatitis (n = 1), inflammatory bowel disease (n = 1), asthma (n = 1), chronic rhinitis (n = 1), allergy (n = 1), and tooth resorption (n = 1).
Assay performance
Mean within assay CV was 26.7% for low, 11.5% for medium and 10.1% for high concentrations, respectively. Mean within-assay CV for all analyzed 10 samples was 15.5%. Mean between-assay CV was 7.6% for concentrations 5.9–30.1 pg/mg. To determine recovery after dilution, a pooled sample with known concentration of cortisol (25.9 pg/mg) was diluted 1:1/3, 1:2, 1:4, 1:8, 1:16 and 1:32. Recovery after dilution was adequately linear, and was 109%–460%, where the most diluted sample represented the highest recovery percentage. Recovery upon addition was 80%-85% for concentrations 8.5–134 pg/mg.
A higher consistency was seen between original and re-run frozen reconstituted samples, compared to original samples and samples prepared from hair that had been stored in aluminum foil, see Table 2.
Hair samples and hair sampling location and sampling method
A total of 255 hair samples were collected (see Fig. 1). Median HCC in clipped samples from the front leg was 5.6 pg/mg (IQR 4–7.2, n = 109), in clipped samples from the abdomen 4.8 pg/mg (IQR 3.4–8, n = 26) and in samples obtained through combing 7.2 pg/mg (IQR 4.8–11.5, n = 120). The HCC range was widest in samples obtained from combing (2.4–240 pg/mg) compared to front leg (1.4–134 pg/mg) and abdomen (1.6–13.6 pg/mg).
Twenty-three cats had hair sampled from both the front leg and from the abdomen, with a Spearman´s Rho of 0.72 (CI 0.39–0.89, n = 23). In Bland–Altman analysis, the mean difference between paired HCC was 0.38 pg/mg and the limits of agreement was -6.74 and 7.50 (LOA, 0.38 ± 1.96 SD). One observation was beyond LOA. Agreement between measurements was higher for lower HCC concentrations (mean ≤ 5.6 pg/mg) than for higher concentrations, see Fig. 2.
Sixty-two cats had hair sampled from both the front leg and by combing, with a Spearman´s Rho of 0.61 (CI 0.41–0.76, n = 62). For the Bland Altman plot, three observational outliers were removed – see Fig. 3. The mean difference in paired values was -5.51 pg/mg and the limits of agreement was -28.05 and 17.02 (LOA, -5.51 ± 1.96 SD, n = 59). Three observations were beyond LOA. As for the comparison between clipped front leg and abdominal samples, agreement between measurements was highest in lower HCC concentrations (mean ≤ 10 pg/mg) and decreased with increasing HCC.
HCC and associations with cat characteristics, health status and stress
Regression analysis with HCC quantified from clipped front leg samples comprised every included cat with front leg samples (n = 109). From univariable linear regression models, sex (p = 0.18) and multi-cat household (p = 0.04), were included in building the multiple linear regression model. Further, the variables health (p = 0.228), presence of other animal species in the household (p = 0.750), occurrence of potential stressors (p = 0.503), and presence of signs of stress (p = 0.270), were included 35,43. In the final linear multivariable regression model, the only factor remaining significant for prediction of HCC were sex and multi-cat household. Females that had experienced pregnancy or heat had higher HCC than spayed females (p = 0.008), and cats that lived in a household with at least one more cat had higher HCC than cats in single cat household (p = 0.042). See Table 3. and 4 for details.
Regression analysis with HCC quantified from combed hair samples comprised every included cat with combed samples (n = 120). From univariable linear regression models, sex (p = 0.102) and health (p = 0.190), were included in building the multiple linear regression model. As previously mentioned, the variables presence of other animal species in the household (p = 0.737), occurrence of potential stressors (p = 0.311), and presence of signs of stress (p = 0.989), were included35,43. In the final linear multivariable regression model, factors remaining significant for prediction of HCC were sex and health. Intact males had higher HCC than spayed females (p = 0.013) and cats with chronic disease had higher HCC than healthy cats (p = 0.025). See Table 3. and 4 for details.
Discussion
This study measured cortisol concentrations in hair from domestic cats, sampled by two different methods and from three different body locations. HCC varied with and within different body locations and sampling methods. Despite this, agreement between methods was found, and both sex and health were associated with HCC. Being a female with experience of pregnancy or estrus or living in a multi-cat household was associated with elevated HCC in hair sampled from the front leg. Presence of chronic disease was associated with increased HCC in combed hair samples, as was being an intact male. Exposure to potential stressors or displaying signs of stress were not associated with changes in HCC. Based on the results of the present study, storing samples as reconstituted samples is preferable for HCC quantification compared to storing hair.
The common practice in hair cortisol analysis involves initially shaving a specific area and then repeating the process after a designated period of hair regrowth, known as the shave-reshave method. This ensures the inclusion of actively growing hairs in the sample, aligning with the time frame of interest, as cortisol is incorporated into actively growing hair strands23,52. Differences in cortisol concentrations among hair from different body regions have been seen in various species22,24,35,53. Therefore, it is recommended to sample hair from one single body region when measuring HCC. In the present study, sampling areas were chosen based on convenience in a clinical setting and to minimize sampling-associated stress for the cat. The aim was to retrospectively study a time frame of 2–16 weeks, with a questionnaire designed to cover the cat’s history during this period in relation to hair sampling.
The specific properties of cat hair have to be considered when using HCC. Hair growth varies with different seasons and photoperiods34,49, factors that were not investigated in the present study. A slower growth rate could result in prolonged cortisol incorporation in the hair, leading to higher concentrations of cortisol, and representing a longer retrospective time for cortisol incorporation. The cat pelage includes primary (guard) and secondary (wool) hairs, and their distribution over the body, their growth rate and follicular activity varies34. These factors, as well as shedding patterns34, and varying hair lengths across different body parts, could have contributed to the variations in HCC seen between different body locations in the present study.
Given the differences in both body location and retrieval method, different HCC between the clipped and combed hair samples was expected. This expectation was confirmed, with the highest variation observed among the combed samples. The influence of a few individuals with very high HCC, predominantly measured from combed samples, could explain some of this variation. Additionally, the broader area of the body from which hair strands are sampled during combing, as opposed to the front leg, may have introduced further variability. Combing involves sampling a large proportion of loose hairs, which could make this method more susceptible to seasonal and individual shedding, and to grooming behaviors. No information on grooming behavior was collected in the present study. Combing may include more skin material in the hair sample, but thorough washing prior to cortisol quantification minimizes external contribution to HCC variability23, and skin material is thus not likely contributing to the wide HCC range with this method. For clipped front leg samples and combed samples, there was consistency in paired measurements, especially for lower HCC. The same pattern of consistency was also evident in paired measurements from hair clipped from the front leg and the abdomen. Similar associations between HCC and cat characteristics and health were found between clipped front leg samples and combed samples, though not all statistically significant at p < 0.050. It is important to note that not all cats were sampled using both methods and from all three locations, and a larger sample size would have been beneficial for comparison. This highlights the challenges faced when collecting samples for research in a clinical setting. Combing is a less standardized method of hair sampling. However, avoiding repeated sampling is beneficial for increasing compliance among cat owners, and hair sampled by combing has potential as an owner- and cat-friendly method of hair sampling for HCC analysis.
Presence of chronic disease was associated with higher HCC, compared to healthy cats. Previously, chronically elevated cortisol levels, as measured in HCC, have been associated with dermatophyte infection in shelter cats43. A higher urine cortisol-creatinine ratio has been described in cats with hyperthyroidism54 and in hospitalized cats with various diseases55, as well as in shelter cats with signs of systemic disease 12. Higher cortisol concentrations could be associated with both the disease process54, and with events that are related to, but not directly associated with disease. This may include veterinary visits and transportation of the cat to the clinic56, cat synchronization with owner stress57, and daily administration of medications. Although hyperthyroidism, chronic kidney disease, and diabetes mellitus were the most commonly represented diseases in the studied population, a diverse range of diseases was observed, and the cats exhibited various stages of illness. Further examination of specific diseases could provide deeper insights into the influence of individual diseases and disease states on HCC. Interestingly, the presence of clinical signs reported by owners did not show an association with HCC. This discrepancy could be attributed to factors such as questionnaire design, inaccurate owner reporting, or the owners’ ability to recognize clinical signs of disease. Furthermore, HPA-axis dysregulation, manifesting as hypocortisolism following a period of chronic HPA-axis hyperactivity, might contribute to this observed discrepancy58. The possibility of a disparity between the observable condition of a chronically ill cat and its actual impact, as measured by HCC, warrants additional investigation.
Our results reveal a sex-associated difference in HCC. In clipped front leg hair, females with reported experiences of pregnancy or estrus, had higher HCC than spayed females. In combed hair, intact males had higher HCC than spayed females. Suggested causes for effect of sex on HCC in different species include the influence of social rank59, territorial behavior60, differences in body condition25, and effect of gonadal steroids on HPA-axis activity 61. During pregnancy, cortisol is crucial for fetal development, leading to increased circulating cortisol concentrations until parturition31,62. A lower HCC has been reported in spayed free-roaming domestic female cats compared to intact females, possibly influenced by agonistic behavior or reproductive status37. The associations in our study may result from hormonal changes during pregnancy, parturition, increased energetic expenditures, and general maternal efforts post-partum38,63. The majority of intact females (15/16) without owner-reported estrus or pregnancy were at least one year old at the time of hair sampling, suggesting potential estrus experiences not reported by the owner 64. In a study of wildcats and feral cats, higher HCC was observed in male cats compared to females, although this difference was not statistically significant, possibly due to a small sample size65. In our study, we found an association between male sex and HCC. However, similar to the previous study, the limited number of cats in our sample influenced the results, with uncertainty highlighted by a wide confidence interval. A larger sample size is necessary for a more comprehensive exploration of HCC and sex in domestic cats.
In the present study, no association between HCC and exposure to potential stressors or display of stress-related behaviors were seen. Previously, HCC in cats has been positively associated to defecating or urinating outside the litterbox35,36 and to displaying aggressive behavior towards family members 36, and negatively associated to a groomed and soft hair coat35. In one of these studies, owner-reported stress levels were not significantly associated with HCC when other factors related to the cat and its home environment were considered 35. An explanation for the lack of association between stress and HCC may be failure of owners to recognize signs of stress in their cats 11, or variability in HPA-axis activity related to individual stressor response 58,66,67. In the present study, no consideration of different cat temperaments and personalities were made, which could affect both stress coping mechanisms and stress responses 3,68.
Cats living in multi-cat households had higher HCC than cats in single cat households. A multi-cat household was defined as the cat sharing living space with at least one other cat, and in the data analysis no differentiation on the total number of cohabitating cats were made. In a multi-cat household, cats need to share resources, with potential emergence of inter-cat aggression and other stress-linked behaviors 69. However, a multitude of potential factors influence cat behavior in both single and multi-cat households 7,46.
This study has several limitations. First, this is an observational study, and as such, there is limited control over the included subjects. The cat population at large is probably well represented, but confounding factors may go undetected. The sample size is small, and the hair sampling performed at home by owners lacked standardization, introducing variability into the collection process. Also, reliance on owner obtained information about the cats may introduce subjectivity and potential inaccuracies, including recall bias and misinterpretations. No clinical confirmation of the cats’ health status or other related information was available. The lack of a validated stress scale or evaluation tool could have limited the accuracy of stress assessment.
Chronic stress has profound implications for feline welfare. Given that stress often goes unnoticed by owners, there is a need for methods to facilitate stress evaluation in cats. The quantification of HCC holds promise for a non-invasive aid in assessment of long-term HPA-axis activity in felines. This study explored a novel, low-stress hair sampling method for cortisol quantification. Although much remains unknown about the factors influencing HCC, samples obtained by combing demonstrate potential for simplified sample collection and assessment of HCC in relation to various cat characteristics. Despite variations between the studied sampling methods, a concordance between them was observed, and associations between HCC and sex and health was seen. Combing showed potential as a cat-friendly sampling method, addressing the challenges encountered in sample collection within a clinical setting. The limitations of this study warrants caution in interpreting results. However, the findings contribute to the potential use of a low-stress combing method for HCC assessment, emphasizing the need for research to further explore this approach.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Moberg, G. P. Biological responses to stress: implications for animal welfare., 1–21 (CABI Publishing, 2000).
Levine, E. D. Feline Fear and Anxiety. Veterinary Clinics of North America: Small Animal Practice38, 1065–1079. https://doi.org/10.1016/j.cvsm.2008.04.010 (2008).
Amat, M., Camps, T. & Manteca, X. Stress in owned cats: behavioural changes and welfare implications. Journal of Feline Medicine and Surgery18, 577–586. https://doi.org/10.1177/1098612x15590867 (2016).
Tanaka, A., Wagner, D. C., Kass, P. H. & Hurley, K. F. Associations among weight loss, stress, and upper respiratory tract infection in shelter cats. Journal of the American Veterinary Medical Association240, 570–576. https://doi.org/10.2460/javma.240.5.570 (2012).
Stella, J. L., Lord, L. K. & Buffington, C. A. T. Sickness behaviors in response to unusual external events in healthy cats and cats with feline interstitial cystitis. Journal of the American Veterinary Medical Association238, 67–73. https://doi.org/10.2460/javma.238.1.67 (2011).
Stella, J., Croney, C. & Buffington, T. Effects of stressors on the behavior and physiology of domestic cats. Applied Animal Behaviour Science143, 157–163. https://doi.org/10.1016/j.applanim.2012.10.014 (2013).
Carlstead, K., Brown, J. L. & Strawn, W. Behavioral and physiological correlates of stress in laboratory cats. Applied Animal Behaviour Science38, 143–158. https://doi.org/10.1016/0168-1591(93)90062-t (1993).
Luescher, A. U. Diagnosis and management of compulsive disorders in dogs and cats. Clinical Techniques in Small Animal Practice19, 233–239. https://doi.org/10.1053/j.ctsap.2004.10.005 (2004).
Cameron, M. E., Casey, R. A., Bradshaw, J. W. S., Waran, N. K. & Gunn-Moore, D. A. A study of environmental and behavioural factors that may be associated with feline idiopathic cystitis. Journal of Small Animal Practice45, 144–147. https://doi.org/10.1111/j.1748-5827.2004.tb00216.x (2004).
Heath, S. E. in The Welfare of Cats (ed I. Rochlitz) Ch. Behaviour problems and welfare, 91–118 (Springer Netherlands, 2007).
Mariti, C. et al. The perception of cat stress by Italian owners. Journal of Veterinary Behavior20, 74–81. https://doi.org/10.1016/j.jveb.2017.04.002 (2017).
McCobb, E. C., Patronek, G. J., Marder, A., Dinnage, J. D. & Stone, M. S. Assessment of stress levels among cats in four animal shelters. Journal of the American Veterinary Medical Association226, 548–555. https://doi.org/10.2460/javma.2005.226.548 (2005).
Broom, D. M. The scientific assessment of animal welfare. Applied Animal Behaviour Science20, 5–19. https://doi.org/10.1016/0168-1591(88)90122-0 (1988).
Davenport, M. D., Tiefenbacher, S., Lutz, C. K., Novak, M. A. & Meyer, J. S. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology147, 255–261. https://doi.org/10.1016/j.ygcen.2006.01.005 (2006).
Meyer, J., Novak, M., Hamel, A. & Rosenberg, K. Extraction and Analysis of Cortisol from Human and Monkey Hair. Journal of Visualized Experimentshttps://doi.org/10.3791/50882 (2014).
Koren, L. et al. A novel method using hair for determining hormonal levels in wildlife. Animal Behaviour63, 403–406. https://doi.org/10.1006/anbe.2001.1907 (2002).
Cirimele, V., Kintz, P., Dumestre, V., Goullé, J. P. & Ludes, B. Identification of ten corticosteroids in human hair by liquid chromatography–ionspray mass spectrometry. Forensic Science International107, 381–388. https://doi.org/10.1016/S0379-0738(99)00180-2 (2000).
González-de-la-Vara, M. D. R. et al. Effects of adrenocorticotropic hormone challenge and age on hair cortisol concentrations in dairy cattle. The Canadian Journal of Veterinary Research75, 216–221 (2011).
Heimbürge, S., Kanitz, E. & Otten, W. The use of hair cortisol for the assessment of stress in animals. General and Comparative Endocrinology270, 10–17. https://doi.org/10.1016/j.ygcen.2018.09.016 (2019).
Russell, E., Koren, G., Rieder, M. & Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology37, 589–601. https://doi.org/10.1016/j.psyneuen.2011.09.009 (2012).
Yumi Yamanashi, M. T. Naruki Morimura, Satoshi Hirata, Juri Suzuki, Misato Hayashi, Kodzue Kinoshita, Miho Murayama, Gen’ichi Idani Analysis of hair cortisol levels in captive chimpanzees: Effect of various methods on cortisol stability and variability. MethodsX3, 110–117. https://doi.org/10.1016/j.mex.2016.01.004 (2016).
Macbeth, B. J., Cattet, M. R. L., Stenhouse, M. L., Gibeau, K. L. & Janz, D. M. Hair cortisol concentration as a noninvasivemeasure of long-term stress in free-ranginggrizzly bears (Ursusarctos): considerations withimplications for other wildlife. Can. J. Zool.88, 935–949 (2010).
Meyer, J. S. & Novak, M. A. Minireview: Hair Cortisol: A Novel Biomarker of Hypothalamic-Pituitary-Adrenocortical Activity. Endocrinology153, 4120–4127. https://doi.org/10.1210/en.2012-1226 (2012).
Carlitz, E. H. D. et al. Measuring Hair Cortisol Concentrations to Assess the Effect of Anthropogenic Impacts on Wild Chimpanzees (Pan troglodytes). PLOS ONE11, e0151870. https://doi.org/10.1371/journal.pone.0151870 (2016).
Cattet, M., Macbeth, B. J., Janz, D. M., Zedrosser, A., Swenson, J. E., Dumond, M., Stenhouse, G. B. . Quantifying long-term stress in brown bears with the hair cortisol concentration: a biomarker that may be confounded by rapid changes in response to capture and handling. Conservation Physiology2, https://doi.org/10.1093/conphys/cou026 (2014).
Ito, N. et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. The FASEB Journal19, 1332–1334. https://doi.org/10.1096/fj.04-1968fje (2005).
Salaberger, T. et al. Influence of external factors on hair cortisol concentrations. General and Comparative Endocrinology233, 73–78. https://doi.org/10.1016/j.ygcen.2016.05.005 (2016).
Bennett, A. & Hayssen, V. Measuring cortisol in hair and saliva from dogs: coat color and pigment differences. Domestic Animal Endocrinology39, 171–180. https://doi.org/10.1016/j.domaniend.2010.04.003 (2010).
Burnett, T. A. et al. Short communication: Factors affecting hair cortisol concentrations in lactating dairy cows. Journal of Dairy Science97, 7685–7690. https://doi.org/10.3168/jds.2014-8444 (2014).
Roth, L. S. V., Faresjö, Å., Theodorsson, E. & Jensen, P. Hair cortisol varies with season and lifestyle and relates to human interactions in German shepherd dogs. Scientific Reports6, 19631. https://doi.org/10.1038/srep19631 (2016).
Bacci, M. L. et al. Hair cortisol determination in sows in two consecutive reproductive cycles. Reproductive Biology14, 218–223. https://doi.org/10.1016/j.repbio.2014.06.001 (2014).
Sharpley, C. F., Kauter, K. G. & McFarlane, J. R. An Investigation of Hair Cortisol Concentration Across Body Sites and within Hair Shaft. Clinical Medicine Insights3, 17–23 (2010).
Ryder, M. L. Seasonal changes in the coat of the cat. Research in Veterinary Science21, 280–283 (1976).
Baker, K. P. Hair growth and replacement in the cat. British Veterinary Journal130, 327–335. https://doi.org/10.1016/S0007-1935(17)35835-9 (1974).
Contreras, E. T., Vanderstichel, R., Hovenga, C. & Lappin, M. R. Evaluation of hair and nail cortisol concentrations and associations with behavioral, physical, and environmental indicators of chronic stress in cats. Journal of Veterinary Internal Medicinehttps://doi.org/10.1111/jvim.16283 (2021).
Wojtaś, J. Hair cortisol levels in cats with and without behavioural problems. Journal of Feline Medicine and Surgery25, 1098612X221150624, https://doi.org/10.1177/1098612X221150624 (2023).
Finkler, H. & Terkel, J. Cortisol levels and aggression in neutered and intact free-roaming female cats living in urban social groups. Physiology & Behavior99, 343–347. https://doi.org/10.1016/j.physbeh.2009.11.014 (2010).
Alekseeva, G. S., Loshchagina, J. A., Erofeeva, M. N. & Naidenko, S. V. Stressed by Maternity: Changes of Cortisol Level in Lactating Domestic Cats. Animals10, 903. https://doi.org/10.3390/ani10050903 (2020).
Arhant, C., Wogritsch, R. & Troxler, J. Assessment of behavior and physical condition of shelter cats as animal-based indicators of welfare. Journal of Veterinary Behavior10, 399–406. https://doi.org/10.1016/j.jveb.2015.03.006 (2015).
Carney, H. C. et al. 2016 AAFP Guidelines for the Management of Feline Hyperthyroidism. Journal of Feline Medicine and Surgery18, 400–416. https://doi.org/10.1177/1098612x16643252 (2016).
Greco, D. S. Diagnosis of Diabetes Mellitus in Cats and Dogs. Veterinary Clinics of North America: Small Animal Practice31, 845–853. https://doi.org/10.1016/S0195-5616(01)50002-9 (2001).
Vogelnest, L. J. Skin as a marker of general feline health: Cutaneous manifestations of systemic disease. Journal of Feline Medicine and Surgery19, 948–960. https://doi.org/10.1177/1098612X17723246 (2017).
Galuppi, R. et al. Cortisol levels in cats’ hair in presence or absence of Microsporum canis infection. Research in Veterinary Science95, 1076–1080. https://doi.org/10.1016/j.rvsc.2013.07.023 (2013).
Accorsi, P. A. et al. Cortisol determination in hair and faeces from domestic cats and dogs. General and Comparative Endocrinology155, 398–402. https://doi.org/10.1016/j.ygcen.2007.07.002 (2008).
Percie Du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLOS Biology18, e3000410, https://doi.org/10.1371/journal.pbio.3000410 (2020).
I, R. Feline welfare issues. 3rd edn, 131–154 (Cambridge University Press, 2013).
Deborah L. Duffy, R. T. D. d. M., James A. Serpell. Development and evaluation of the Fe-BARQ: A new survey instrument for measuring behavior in domestic cats (Felis s. catus). Behavioural Processes141, 329–341, https://doi.org/10.1016/j.beproc.2017.02.010 (2017).
Heath, S. E. 91–118 (Springer, 2007).
Hendriks, W. H., Tarttelin, M. F. & Moughan, P. J. Seasonal Hair Growth in the Adult Domestic Cat (Felis catus). Comparative Biochemistry and Physiology Part A: Physiology116, 29–35. https://doi.org/10.1016/s0300-9629(96)00113-2 (1997).
RStudio: Integrated Development Environment for R (2022).
Minitab v. 19.2020.1 (2020).
Gow, R., Thomson, S., Rieder, M., Van Uum, S. & Koren, G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Science International196, 32–37. https://doi.org/10.1016/j.forsciint.2009.12.040 (2010).
Terwissen, C. V., Mastromonaco, G. F. & Murray, D. L. Influence of adrenocorticotrophin hormone challenge and external factors (age, sex, and body region) on hair cortisol concentration in Canada lynx (Lynx canadensis). General and Comparative Endocrinology194, 162–167. https://doi.org/10.1016/j.ygcen.2013.09.010 (2013).
De Lange, M. S., Galac, S., Trip, M. R. J. & Kooistra, H. S. High Urinary Corticoid/Creatinine Ratios in Cats with Hyperthyroidism. Journal of Veterinary Internal Medicine18, 152–155. https://doi.org/10.1111/j.1939-1676.2004.tb00154.x (2004).
Henry, C. J., Clark, T. P., Young, D. W. & Spano, J. S. Urine Cortisol: Creatinine Ratio in Healthy and Sick Cats. Journal of Veterinary Internal Medicine10, 123–126. https://doi.org/10.1111/j.1939-1676.1996.tb02043.x (1996).
Quimby, J. M., Smith, M. L. & Lunn, K. F. Evaluation of the Effects of Hospital Visit Stress on Physiologic Parameters in the Cat. Journal of Feline Medicine and Surgery13, 733–737. https://doi.org/10.1016/j.jfms.2011.07.003 (2017).
Sundman, A.-S. et al. Long-term stress levels are synchronized in dogs and their owners. Scientific Reports9, https://doi.org/10.1038/s41598-019-43851-x (2019).
Packer, R. M. A. et al. What can we learn from the hair of the dog? Complex effects of endogenous and exogenous stressors on canine hair cortisol. PLOS ONE14, e0216000. https://doi.org/10.1371/journal.pone.0216000 (2019).
Lafferty, D. J. R., Laudenslager, M. L., Mowat, G., Heard, D. & Belant, J. L. Sex, Diet, and the Social Environment: Factors Influencing Hair Cortisol Concentration in Free-Ranging Black Bears (Ursus americanus). PLOS ONE10, e0141489. https://doi.org/10.1371/journal.pone.0141489 (2015).
Azevedo, A. et al. Age, sex and storage time influence hair cortisol levels in a wild mammal population. PLOS ONE14, e0221124. https://doi.org/10.1371/journal.pone.0221124 (2019).
Laudenslager, M. L., Jorgensen, M. J. & Fairbanks, L. A. Developmental patterns of hair cortisol in male and female nonhuman primates: Lower hair cortisol levels in vervet males emerge at puberty. Psychoneuroendocrinology37, 1736–1739. https://doi.org/10.1016/j.psyneuen.2012.03.015 (2012).
Braun, U. C., G ; Baumgartner, M R ; Riond, Barbara ; Binz, T M. Hair cortisol concentration and adrenal gland weight in healthy and ill cows. Schweizer Archiv für Tierheilkunde159, 493–495, https://doi.org/10.17236/sat00128 (2017).
Fusi, J. et al. How Stressful Is Maternity? Study about Cortisol and Dehydroepiandrosterone-Sulfate Coat and Claws Concentrations in Female Dogs from Mating to 60 Days Post-Partum. Animals11, 1632. https://doi.org/10.3390/ani11061632 (2021).
Griffin, B. Profilic Cats: The Estrous Cycle. Compendium23, 1049–1057 (2001).
Franchini, M. et al. Cortisol in hair: a comparison between wild and feral cats in the north-eastern Alps. European Journal of Wildlife Research65, https://doi.org/10.1007/s10344-019-1330-2 (2019).
Miller, G. E., Chen, E. & Zhou, E. S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological bulletin133, 25 (2007).
Stella, J. & Croney, C. Coping styles in the domestic cat (Felis silvestris catus) and implications for cat welfare. Animals9, 370 (2019).
McCune, S. The impact of paternity and early socialisation on the development of cats’ behaviour to people and novel objects. Applied Animal Behaviour Science45, 109–124. https://doi.org/10.1016/0168-1591(95)00603-P (1995).
Elzerman, A. L., Deporter, T. L., Beck, A. & Collin, J.-F. Conflict and affiliative behavior frequency between cats in multi-cat households: a survey-based study. Journal of Feline Medicine and Surgery22, 705–717. https://doi.org/10.1177/1098612x19877988 (2020).
Acknowledgements
Funding provided by the Greater Stockholm Veterinary Hospital Foundation and The Maj Johnson Fund at the Swedish University of Agriculture (SLU). This work was possible thanks to the Greater Stockholm Veterinary Hospital Foundation and DVM Erika Brandeker at the Regional Small Animal Hospital Anicura Bagarmossen. The authors wish to thank all cats, owners, and animal clinic staff participating in the study, Karolina Engdahl (Department of Clinical Sciences, SLU) for statistical support, and Lena-Mari Tamminen and Anna Svensson (Department of Clinical Sciences, SLU) for assistance with running the hair cortisol analyses.
Funding
Open access funding provided by Swedish University of Agricultural Sciences. The Greater Stockholm Veterinary Hospital Foundation,1-2022,1-2022,1-2022,1-2022,1-2022,1-2022,1-2022,1-2022,Maj Johnson Fund.
Author information
Authors and Affiliations
Contributions
The sampling was performed by NRZ, EJ, FJB, and SS. Data collection and laboratory analyses were performed by NRZ, EJ, and FJB. NRZ analyzed the data and wrote the main manuscript, in collaboration with HR, MÖ, CM, and BSH. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rothlin-Zachrisson, N., Röcklinsberg, H., Jettel, E. et al. Hair cortisol concentrations in clipped and combed hair and associations with characteristics, health status and stress in domestic cats. Sci Rep 14, 21846 (2024). https://doi.org/10.1038/s41598-024-73226-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73226-w
- Springer Nature Limited